Abstract
Introduction
Increased heterogeneity in ventricular repolarization is a risk factor of sudden cardiac death, but its natural history is unclear. Here we examined whether insulin resistance is associated with longitudinal change in ventricular repolarization heterogeneity in apparently healthy subjects.
Methods
The study subjects were participants in health checkups in cohort 1 and cohort 2, which were followed up for 6 years and 5 years, respectively. Subjects with diabetes, cardiovascular disease, or renal disease at baseline were excluded from the analyses. As indices of insulin resistance, the homeostasis model assessment of insulin resistance (HOMA-IR) and triglyceride to HDL-cholesterol ratio (TG/HDL-C) were used in cohort 1 and cohort 2, respectively. Heterogeneity in ventricular repolarization was assessed by heart rate-corrected Tpeak–Tend interval in V5 (cTpTe), QT interval, and QT dispersion. In regression analyses, parameters with a skewed distribution were normalized by logarithmic transformation or by Box–Cox transformation.
Results
In longitudinal analyses, Box–Cox-transformed cTpTe at the end of follow-up was weakly correlated with log HOMA-IR at baseline in cohort 1 (n = 153, r = − 0.207, 95% CI − 0.354 to − 0.050, p = 0.010) and with log TG/HDL-C at baseline in cohort 2 (n = 738, r = − 0.098, 95% CI − 0.169 to − 0.026, p = 0.008). Multiple regression analysis showed that indices of insulin resistance, but not glycosylated hemoglobin (HbA1c) or plasma glucose, at baseline were significant explanatory variables for cTpTe at the end of follow-up. Neither QT interval nor QT dispersion was correlated with metabolic parameters.
Conclusion
Insulin resistance may be involved in the longitudinal increase of ventricular repolarization heterogeneity in apparently healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of patients with diabetes mellitus has increased by fourfold worldwide in the past three decades [1], indicating expansion of the global risk of cardiovascular events. One of the phenotypes of diabetes-associated cardiovascular events is sudden cardiac death (SCD), and its mechanism is multifactorial, including acceleration of atherosclerosis, impairment of ventricular contractile function, and increased susceptibility to ventricular arrhythmias [2,3,4,5]. Pathological alterations in ventricular conduction and repolarization caused by diabetes are reflected by prolongation of the corrected QT (QTc) interval, increase in QT dispersion (QTd), and/or prolongation of the Tpeak–Tend interval (TpTe) in an electrocardiogram (ECG) [5,6,7,8,9,10]. Multiple factors are potentially involved in the abnormality of ventricular repolarization depending on the severity of diabetes and treatment [11,12,13,14]. However, the natural history of cardiac repolarization abnormality in patients with diabetes or other metabolic derangements is still unclear.
In the present study, we hypothesized that insulin resistance is associated with longitudinal increase in cardiac repolarization heterogeneity in an apparently healthy population. To examine this hypothesis, we determined relationships between indices of insulin resistance and longitudinal changes in indices of cardiac repolarization heterogeneity in apparently healthy subjects in two cohorts that we have prospectively followed.
Methods
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Sapporo Medical University and was conducted in strict adherence with the principles of the Declaration of Helsinki. Written informed consent was obtained from all study subjects.
Study Subjects
We retrieved data from two cohorts, the Tanno-Sobetsu cohort [15, 16] and Maruyama Clinic cohort [17], in both of which data of annual health checkups have been prospectively collected. Since storage periods of ECGs were 6 years and 5 years after the health checkups in the Tanno-Sobetsu cohort and Maruyama Clinic cohort, respectively, we selected those periods as follow-up periods in the present study.
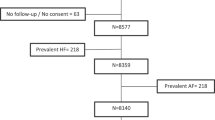
Cohort 1 (Tanno-Sobetsu cohort): Subjects in this cohort were residents of Tanno and Sobetsu, rural towns in Japan. We retrieved data for 346 Sobetsu residents who received health checkups in both 2008 and 2014. To exclude effects of pre-existing morbidities on ventricular repolarization, we excluded subjects who met one or more of the following criteria: diabetes mellitus, regular use of medications for cardiovascular and/or metabolic diseases, hypo- or hyperkalemia (serum K+ < 3.5 or > 5.5 mEq/L), chronic kidney disease at stage 4 or 5, and electrocardiographic abnormalities that modify indices of ventricular repolarization (such as bundle branch block, WPW syndrome, and atrial fibrillation). Diabetes mellitus, which was defined as a history of diabetes mellitus, fasting plasma glucose (FPG) level ≥ 126 mg/dl (7 mmol/L), glycosylated hemoglobin (HbA1c) level ≥ 6.5%, and/or use of medication for diabetes mellitus, was diagnosed at baseline and at the end of the follow-up. In accordance with the exclusion criteria, 193 subjects were excluded, and data for 153 subjects in cohort 1 contributed to analyses.
Cohort 2 (Maruyama Clinic cohort): Subjects in this cohort were those who underwent medical health checkups at a clinic located in Sapporo, an urban area in Japan. We retrieved data for 1004 subjects who received medical checkups in both 2009 and 2014. The same exclusion criteria as those in cohort 1 were applied in this cohort, and 266 subjects were excluded. Data for the remaining 738 subjects in this cohort were used for analyses.
Demographic and Laboratory Examinations
Cohort 1: Medical checkups, including venous blood sampling, were conducted in the early morning after an overnight fast. Information regarding past history of diseases and medication was collected by public health nurses or clinic nurses on the basis of an interview form. Waist circumference (WC), body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured by trained nurses. LDL-cholesterol (LDL-C) was calculated by the Friedewald equation. HbA1c was expressed on the National Glycohemoglobin Standardization Program (NGSP) scale. Serum insulin level was determined by an enzyme immunoassay, and the homeostasis model assessment of insulin resistance (HOMA-IR), an index of insulin sensitivity, was calculated by using following the equation: HOMA-IR = fasting glucose (mg/dl) × fasting insulin (mU/L)/405 [18].
Cohort 2: Medical checkups including measurements of demographic parameters, blood pressure measurements, and analyses of venous blood samples were similar to those in cohort 1. Since serum insulin level was not determined in cohort 2, triglyceride (TG)/HDL-cholesterol (HDL-C) was calculated as an index of insulin resistance [19, 20].
ECG Analysis
Standard 12-lead ECGs at rest were recorded using an electrocardiograph FCP-3610 (Fukuda Denshi Co., Ltd. Tokyo, Japan) in cohort 1 and by using CardioFaxV ECG-1550 (Nihon Kohden Co., Ltd. Tokyo, Japan) in cohort 2. Since both electrocardiographs had passed Japanese Industrial Standards, significant data variability by the electrocardiographs was unlikely. As indices of ventricular repolarization heterogeneity, three currently used indices were measured: QTc, QTd, and TpTe corrected by heart rate (cTpTe) [21]. The QT intervals were measured in all 12 leads and corrected for heart rate by Bazett’s formula. QTd was calculated as the difference between the longest and shortest QT intervals among the 12 leads. TpTe was measured in milliseconds (ms) from the peak of the T wave to the intersection between the tangent at the steepest point of the T wave downslope and the isoelectronic line at lead V5 [22]. Since correction of TpTe by heart rate using the Bazett or Fridericia formula has been shown to improve the predictive value of TpTe as a marker of sudden cardiac death [23], we calculated cTpTe by using Bazett’s formula. ECG parameters in all study subjects were measured manually by a single investigator (T. Matsumoto), a board-certified cardiologist. Accuracy of her measurement of ECG indices had been confirmed by two co-authors using 10 randomly selected cases of ECG.
Statistical Analysis
Numeric variables are expressed as mean ± SD for data showing a normal distribution or medians and interquartile ranges for data showing a skewed distribution. Normality of data distribution was examined by the Shapiro–Wilk test. Differences in parameters between the two groups were examined by Student’s t test, Mann–Whitney U test, or Chi-square test depending on the parameter and its distribution. The relationships between parameters were examined using simple and multiple linear regression analyses. Data with a skewed distribution were normalized by logarithmic transformation or by Box–Cox transformation [24] to make them usable for the regression analyses as in previous studies [25, 26]. Correlation of parameters was examined by use of Pearson’s correlation coefficient. Multiple linear regression analyses were conducted by using the forced entry method to examine relationships between indices of insulin resistance at baseline and indices of ventricular repolarization at the end of follow-up. On the basis of current knowledge regarding insulin resistance and cardiac electrical remodeling [20, 27], we selected age, sex, BMI, and SBP as confounding factors of insulin resistance in the regression analyses. For all tests, p < 0.05 was considered statistically significant. All data were analyzed by using JMP11 for Windows (SAS Institute, Cary, NC, USA).
Results
Cross-Sectional Analysis of Data in Cohort 1 (Tanno-Sobetsu Cohort)
Baseline characteristics of the study subjects (data in 2008) are shown in Table 1. The mean age of the subjects was 58.4 years and 46.4% of them were men. BMI, WC, DBP, and percentage of subjects with a smoking habit were higher in men than in women. FPG, HOMA-IR, and serum TG were higher and HDL-C was lower in men than in women.
Mean QTc interval was shorter and SV1 + RV5 was larger in men than in women, whereas heart rate, QTd, TpTe, and cTpTe were comparable in men and women. HOMA-IR, cTpTe, and QTd were transformed into log HOMA-IR, 1/√cTpTe, log QTd (2008), and √QTd (2014), respectively, for parametric analyses. SBP was correlated with 1/√cTpTe (r = − 0.162, 95% confidence interval [CI] − 0.313 to − 0.004, p = 0.045), and DBP was correlated with log QTd (r = − 0.185, 95% CI − 0.334 to − 0.027, p = 0.022) and 1/√cTpTe (r = − 0.199, 95% CI − 0.346 to − 0.041, p = 0.014). There was a weak correlation between log HOMA-IR and 1/√cTpTe (r = − 0.159, 95% CI − 0.319 to − 0.001, p = 0.049). However, neither FPG nor HbA1c was correlated with 1/√cTpTe. Log QTd was not correlated with log HOMA-IR, FPG, HbA1c, or serum lipid levels (data not shown).
Insulin resistance defined by HOMA-IR ≥ 1.73 [28] was observed in 16.3% (25/153) of the study subjects at baseline, and their indices of ventricular repolarization heterogeneity are summarized in Table 2. Baseline cTpTe and QTd were not significantly different between subjects with HOMA-IR ≥ 1.73 and those with HOMA-IR < 1.73.
Longitudinal Analysis of Data in Cohort 1
To determine factors that predict longitudinal changes in QTd and cTpTe, we examined correlations between clinical and laboratory variables in 2008 and √QTd and 1/√cTpTe in 2014. There was a weak correlation between log HOMA-IR at baseline and 1/√cTpTe in 2014 (r = − 0.207, 95% CI − 0.354 to − 0.050, p = 0.010) (Fig. 1), whereas such a correlation was not found between baseline FPG or HbA1c and 1/√cTpTe in 2014. √QTd in 2014 was not correlated with log HOMA-IR, HbA1c, or FPG at baseline (data not shown).
As shown in Table 3, multiple linear regression analysis for 1/√cTpTe in 2014 revealed that log HOMA-IR at baseline was an independent explanatory variable of 1/√cTpTe after adjustment for age and sex (model 1), and the relation was still significant after additional adjustment for SBP and BMI (model 2 and model 3). In contrast, FPG and HbA1c at baseline were not significant explanatory variables of 1/√cTpTe in 2014 in any of the models.
Cross-Sectional Analysis of Data in Cohort 2 (Maruyama Clinic Cohort)
As shown in Table 4, the mean age of the subjects was 46.0 years and 57.0% were men. Mean age, BMI, WC, SBP, DBP, FPG, TG, LDL-C, TG/HDL-C ratio, and percentage of subjects with a smoking habit were higher in men than in women, and HDL-C was lower in men.
In electrocardiographic variables, HR and QTd at baseline were slightly but significantly lower in men than in women in this cohort. Mean QTc interval was shorter and SV1 + RV5 was larger in men than in women and there was no statistical difference in TpTe and cTpTe between men and women at baseline, being consistent with data in cohort 1.
In cohort 2, TG/HDL-C, cTpTe, and QTd were transformed into log TG/HDL-C, 1/cTpTe, and √QTd, respectively, for parametric analyses. SBP (r = − 0.134, 95% CI − 0.204 to − 0.062, p = 0.0003), DBP (r = − 0.139, 95% CI − 0.209 to − 0.068, p = 0.0001), log TG (r = − 0.087, 95% CI − 0.158 to − 0.015, p = 0.019), and log TG/HDL (r = − 0.083, 95% CI − 0.154 to − 0.011, p = 0.025), but not FPG or HbA1c, were weakly correlated with 1/cTpTe at baseline. √QTd was not correlated with any of the clinical or metabolic parameters.
Insulin resistance defined by TG/HDL-C ≥ 3 [19] was observed in 16.4% of the study subjects at baseline, and their indices of ventricular repolarization heterogeneity are summarized in Table 5. Baseline cTpTe and QTd were not significantly different between subjects with TG/HDL-C ≥ 3 and those with TG/HDL < 3.
Longitudinal Analysis of Data in Cohort 2
As shown in Fig. 2, 1/cTpTe in 2014 was very weakly correlated with log TG/HDL-C at baseline (r = − 0.098, 95% CI − 0.169 to − 0.026, p = 0.008) and with HbA1c at baseline (r = − 0.084, 95% CI − 0.156 to − 0.012, p = 0.022), whereas such a correlation was not found between baseline FPG and 1/cTpTe in 2014. √QTd in 2014 was not correlated with log TG/HDL-C, HbA1c, or FPG at baseline (data not shown).
Relationships between TG/HDL-C in 2009, HbA1c in 2009, and cTpTe in 2014: cohort 2. Because of the non-normalized distribution of data, TG/HDL-C and cTpTe data were normalized to log TG/HDL-C and to 1/cTpTe, respectively. a y = 12.937–0.246 x, r = − 0.098 (95% CI − 0.169 to − 0.026), p = 0.008; b y = 15.403–0.532 x, r = − 0.084 (95% CI − 0.156 to − 0.012), p = 0.022
In multiple linear regression analysis for 1/cTpTe in 2014 (Table 6), log TG/HDL-C at baseline was found to be an independent explanatory variable after adjustment for age and sex (model 1). The association of log TG/HDL-C at baseline with 1/cTpTe in 2014 was significant after additional adjustment for SBP and BMI (Table 6, model 2 and model 3). In contrast, HbA1c at baseline was not selected as an independent explanatory variable of 1/cTpTe in 2014 after adjustment for age and sex. There was no significant relation between √QTd in 2014 and TG/HDL-C ratio, HbA1c, or FPG at baseline (data not shown).
Discussion
In addition to weak correlations between indices of insulin resistance and cTpTe at baseline in both cohort 1 and cohort 2 in the cross-sectional analyses, a significant correlation was found between an index of insulin resistance at baseline and cTpTe in 2014. Furthermore, results of multiple linear regression analyses of data in the two cohorts (Tables 3 and 6) consistently indicated that insulin resistance assessed by HOMA-IR or TG/HDL-C was independently associated with increase in cTpTe after 5–6 years of follow-up. Correlation coefficients in the longitudinal relationship were small in both cohorts (Figs. 1 and 2), but it is not unexpected since we excluded subjects on regular medications, diabetic patients, and patients with chronic kidney disease from the study subjects for the analysis. Ranges of data for indices of insulin resistance and cTpTe in the study subjects were not largely deviated from “normal” ranges both at baseline and during the follow-up, which may have made it difficult to detect an association of pathological changes in the two factors. Nevertheless, earlier studies have shown that abnormality of ventricular repolarization in obese subjects and patients with metabolic syndrome is significantly improved by long-term exercise and weight loss together with improvement of insulin sensitivity [29, 30]. Together with the earlier findings, the results of the present study suggest that insulin resistance plays a role in the increase in cardiac repolarization heterogeneity in apparently healthy subjects.
To use univariable and multivariable regression analyses, we normalized data for QTd and cTpTe by Box–Cox transformation and we normalized data for HOMA-IR and TG/HDL-C by logarithmic transformation as in earlier studies [25, 26]. Because of such transformations, the extent of change in cTpTe by the level of HOMA-IR or TG/HDL-C is not easily observable in Figs. 1 and 2. However, calculation using regression equations indicated that the median and 90th percentile values of HOMA-IR, 1.04 and 2.14, at baseline predict cTpTe of 78.2 and 82.6 ms, respectively, 6 years later in cohort 1. Similarly, the median and 90th percentile values of TG/HDL-C, 1.32 and 3.93, predict cTpTe of 77.7 and 79.4 ms, respectively, 5 years later in cohort 2. cTpTe data for the subjects with 90th percentile HOMA-IR and TG/HDL-C are actually close to the average cTpTe in diabetic patients in earlier studies (i.e., 76.5–86.0 ms) [9, 10, 14, 31]. The modest impact of change in an index of insulin resistance on cTpTe prolongation (Figs. 1 and 2), as compared with the impact of diabetes, rather supports the notion that insulin resistance is the earliest causal factor of multiple factors involved in abnormalities of cardiac repolarization in diabetic patients.
In contrast to TpTe, QTd was not correlated with indices of insulin resistance in the two cohorts. QTd indicates the difference in action potential durations between two regions, and TpTe is an index of global dispersion of repolarization [20, 32]. Both QTd and TpTe have been shown to increase in patients with diabetes mellitus [6,7,8,9,10]. Obesity and metabolic syndrome are also reportedly associated with an increase in QTd [29, 30], though an increase in TpTe caused by obesity or metabolic syndrome, to our knowledge, has not been reported. Taken together, the findings suggest that dispersion of repolarization by insulin resistance precedes an increase in heterogeneity of action potential duration (APD) by hyperglycemia.
During the 6-year follow-up in cohort 1 and the 5-year follow-up in cohort 2, 3.3% (n = 5) and 2.3% (n = 17) of the subjects, respectively, newly developed diabetes mellitus. Since the number of patients with new-onset diabetes in each cohort was small, it was difficult to statistically determine the relationship between new-onset diabetes and change in QTd or cTpTe.
The mechanism by which insulin resistance induces abnormality of ventricular repolarization has been studied by animal experiments. Phosphoinositide 3-kinase (PI3K), which is coupled with the insulin receptor, has been shown to regulate multiple ion channels involved in repolarization of cardiomyocytes [33]. Knockout of the insulin receptors in cardiomyocytes suppressed protein expression of Kv4.2 and KChIP2, which reduced transient outward K+ current (Ito current), leading to prolongation of APD and QT on a surface ECG [34]. Similar reduction of Kv4.2 and KChIP2 and APD prolongation were observed in cardiomyocytes of a rat model of type 2 diabetes with hyperinsulinemia and insulin resistance [13]. However, since Ito current is smaller in human cardiomyocytes than in rodent cardiomyocytes, the findings in rat hearts may not be extrapolated to patients with insulin resistance. Increase in sympathetic tone might also be involved in increased heterogeneity of ventricular repolarization in association with insulin resistance [35, 36]. In addition, in diabetic patients, diabetes-related comorbidities such as coronary artery disease and cardiac hypertrophy are possibly involved in the abnormality of ventricular repolarization depending on the severity and duration of diabetes.
Study Limitations
There are limitations in the present study. First, since different indices of insulin resistance and different equations for Box–Cox transformation were used in the two cohorts, we could not directly compare the changes in cTpTe caused by insulin resistance in cohort 1 and cohort 2. Additionally, we cannot exclude the possibility that HDL-C [37] and/or LDL-C particle size, which is also reflected by TG/HDL-C [38], were responsible for the change in cTpTe during follow-up. Second, since follow-up periods were relatively short, the impact of a slight change in insulin sensitivity on a long natural history of ventricular repolarization remains unclear. Third, because a single investigator measured ECG parameters, ECG measurements might have been biased, though precision of the data appears high because the data are free from interobserver differences.
Conclusions
Longitudinal analyses of two independent cohorts showed that indices of insulin resistance, HOMA-IR and TG/HDL-C, were independent explanatory variables for increase in cTpTe, an index of heterogeneity of ventricular repolarization, during 5- to 6-year follow-up in apparently healthy individuals. The findings support the notion that insulin resistance is the earliest causal factor in abnormal ventricular repolarization in patients with diabetes.
References
WHO. Global report on diabetes. World Health Organization, Geneva. 2016. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Accessed 30 June 2019
Yeung CY, Lam KS, Li SW, Lam KF, Tse HF, Siu CW. Sudden cardiac death after myocardial infarction in type 2 diabetic patients with no residual myocardial ischemia. Diabetes Care. 2012;35:2564–9.
Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–13.
Andersson J, Wennberg P, Lundblad D, Escher SA, Jansson JH. Diabetes mellitus, high BMI and low education level predict sudden cardiac death within 24 hours of incident myocardial infarction. Eur J Prev Cardiol. 2016;23:1814–20.
Ninkovic VM, Ninkovic SM, Miloradovic V, et al. Prevalence and risk factors for prolonged QT interval and QT dispersion in patients with type 2 diabetes. Acta Diabetol. 2016;53:737–44.
Wei K, Dorian P, Newman D, Langer A. Association between QT dispersion and autonomic dysfunction in patients with diabetes mellitus. J Am Coll Cardiol. 1995;26:859–63.
Junttila MJ, Barthel P, Myerburg RJ, et al. Sudden cardiac death after myocardial infarction in patients with type 2 diabetes. Heart Rhythm. 2010;7:1396–403.
Sacre JW, Franjic B, Coombes JS, Marwick TH, Baumert M. QT interval variability in type 2 diabetic patients with cardiac sympathetic dysinnervation assessed by 123I-metaiodobenzylguanidine scintigraphy. J Cardiovasc Electrophysiol. 2013;24:305–13.
Miki T, Tobisawa T, Sato T, et al. Does glycemic control reverse dispersion of ventricular repolarization in type 2 diabetes? Cardiovasc Diabetol. 2014;13:125.
Tokatli A, Kiliçaslan F, Alis M, Yiginer O, Uzun M. Prolonged Tp-e interval, Tp-e/QT ratio and Tp-e/QTc ratio in patients with type 2 diabetes mellitus. Endocrinol Metab (Seoul). 2016;31:105–12.
Khandoker AH, Imam MH, Couderc JP, Palaniswami M, Jelinek HF. QT variability index changes with severity of cardiovascular autonomic neuropathy. IEEE Trans Inf Technol Biomed. 2012;16:900–6.
Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–66.
Sato T, Kobayashi T, Kuno A, et al. Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am J Physiol Heart Circ Physiol. 2014;306:H1054–65.
Sato T, Miki T, Ohnishi H, et al. Effect of sodium-glucose co-transporter-2 inhibitors on impaired ventricular repolarization in people with type 2 diabetes. Diabet Med. 2017;34:1367–71.
Ohnish H, Saitoh S, Takagi S, et al. Incidence of type 2 diabetes in individuals with central obesity in a rural Japanese population: the Tanno and Sobetsu study. Diabetes Care. 2006;29:1128–9.
Mitsumata K, Saitoh S, Ohnishi H, Akasaka H, Miura T. Effects of parental hypertension on longitudinal trends in blood pressure and plasma metabolic profile: mixed-effects model analysis. Hypertension. 2012;60:1124–30.
Akasaka H, Yoshida H, Takizawa H, et al. The impact of elevation of serum uric acid level on the natural history of glomerular filtration rate (GFR) and its sex difference. Nephrol Dial Transpl. 2014;29:1932–9.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–9.
Giannini C, Santoro N, Caprio S, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34:1869–74.
Tse G, Yan BP. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace. 2017;19:712–21.
Antzelevitch C, Sicouri S, Di Diego JM, et al. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–6.
Chua KC, Rusinaru C, Reinier K, et al. Tpeak-to-Tend interval corrected for heart rate: a more precise measure of increased sudden death risk? Heart Rhythm. 2016;13:21181–2185.
Box GEP, Cox DR. An analysis of transformation. J R Stat Soc. 1964;26:211–52.
van der Loo B, Braun J, Koppensteiner R. On-treatment function testing of platelets and long-term outcome of patients with peripheral arterial disease undergoing transluminal angioplasty. Eur J Vasc Endovasc Surg. 2011;42:809–16.
Greaves RF, Pitkin J, Ho CS, Baglin J, Hunt RW, Zacharin MR. Hormone modeling in preterm neonates: establishment of pituitary and steroid hormone reference intervals. J Clin Endocrinol Metab. 2015;100:1097–103.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;31:122.
Ohnishi H, Saitoh S, Ura N, et al. Relationship between insulin resistance and accumulation of coronary risk factors. Diabetes Obes Metab. 2002;4:388–93.
Jani Y, Kamberi A, Xhunga S, et al. The influence of type 2 diabetes and gender on ventricular repolarization dispersion in patients with sub-clinic left ventricular diastolic dysfunction. Am J Cardiovasc Dis. 2015;5:155–66.
Drigny J, Gremeaux V, Guiraud T, Gayda M, Juneau M, Nigam A. Long-term high-intensity interval training associated with lifestyle modifications improves QT dispersion parameters in metabolic syndrome patients. Ann Phys Rehabil Med. 2013;56:356–70.
Omran J, Firwana B, Koerber S, Bostick B, Alpert MA. Effect of obesity and weight loss on ventricular repolarization: a systematic review and meta-analysis. Obes Rev. 2016;17:520–30.
Wellens HJ, Schwartz PJ, Lindemans FW, et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642–51.
Ballou LM, Lin RZ, Cohen IS. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ Res. 2015;116:127–37.
Lopez-Izquierdo A, Pereira RO, Wende AR, Punske BB, Abel ED, Tristani-Firouzi M. The absence of insulin signaling in the heart induces changes in potassium channel expression and ventricular repolarization. Am J Physiol Heart Circ Physiol. 2014;306:H747–54.
Vaseghi M, Yamakawa K, Sinha A, et al. Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. Am J Physiol Heart Circ Physiol. 2013;305:H1020–30.
Yagishita D, Chui RW, Yamakawa K, et al. Sympathetic nerve stimulation, not circulating norepinephrine, modulates T-peak to T-end interval by increasing global dispersion of repolarization. Circ Arrhythm Electrophysiol. 2015;8:174–85.
Kirchhof P, Fabritz L. High-density lipoprotein shortens the ventricular action potential. A novel explanation for how statins prevent sudden arrhythmic death? J Am Coll Cardiol. 2011;58:45–7.
Boizel R, Benhamou PY, Lardy B, Laporte F, Foulon T, Halimi S. Ratio of triglycerides to HDL cholesterol is an indicator of LDL particle size in patients with type 2 diabetes and normal HDL cholesterol levels. Diabetes Care. 2000;23:1679–85.
Acknowledgements
We are very grateful to the participants of the study.
Funding
This study was supported by Grants for Education and Research from Sapporo Medical University 2017 and 2018. Article processing charges were funded by the authors.
Editorial Assistance
We would like to thank Mr. Stewart Chisholm, S.E.S. Translation and Proofreading Services for editing and proofreading this manuscript.
Authorship
All authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, have responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Tamaki Matsumoto, Hirofumi Ohnishi, Tatsuya Sato, Takayuki Miki, Hiroshi Akasaka, Nagisa Hanawa, Masayuki Koyama, Shigeyuki Saitoh, and Tetsuji Miura have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Sapporo Medical University and was conducted in strict adherence with the principles of the Declaration of Helsinki. Written informed consent was obtained from all of the study subjects.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8320205.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Matsumoto, T., Ohnishi, H., Sato, T. et al. Insulin Resistance is Associated with Longitudinal Changes of Cardiac Repolarization Heterogeneity in Apparently Healthy Subjects. Cardiol Ther 8, 239–251 (2019). https://doi.org/10.1007/s40119-019-0140-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-019-0140-7