Abstract
W-doped ZnO nanocomposite (W-ZnO) is easily prepared and characterized using a variety of techniques including XRD, TEM, BET, ICP-OES and SEM. This reagent can be used as an efficient and heterogeneous catalyst for the preparation of biscoumarins in water under mild conditions. Easy preparation of the catalyst, mild reaction conditions, easy work-up procedure, excellent yields and short reaction times are some of the advantages of this work. In addition, in this article and for the first time, the preparation of biscoumarins from the protected derivatives of aldehydes including oximes, hydrazones and 1,1-diacetates is reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Biscoumarins are a large group of heterocycles with diverse, interesting and important biological and pharmaceutical activities [1–5] activities. In recent years and because of these important activities, several methods are reported for the synthesis of biscoumarins using a variety of catalysts and reagents [6–14]. These methods although useful but most of them suffer from disadvantages such as long reaction times (e.g., in the presence of piperidine [4], the reactions are performed during 3–4 h), unsatisfactory yields (e.g., when HBF4 is used as the catalyst the products are obtained in 55–70 % yields [9] ), harsh reaction conditions, expensive reagents, hazardous and toxic solvents (e.g., refluxing toluene is used as the solvent in some of these methodologies [13]) or catalysts and tedious work-up. Therefore, introduction of efficient and economical catalysts that solve these drawbacks is desirable.
Oximes and semicarbazones are used not only for the isolation, purification and characterization but also for the protection of carbonyl compounds [15, 16]. Since oximes can be prepared from non-carbonyl compounds [17–19], the regeneration of carbonyl compounds from oximes provides an alternative method for the preparation of aldehydes and ketones. In addition, oximes can also be used as intermediates for the preparation of nitriles [20–22], nitrones [23], amines [24], amides [25], isoxazoles [26] and chiral α-sulfinyloximes [27]. Because of the remarkable stability of the acylals to neutral and basic conditions, these compounds have been introduced as the other suitable protection group for aldehydes [28]. In addition, they can be converted into other useful functional groups by reaction with appropriate nucleophiles [29] and used as carbonyl surrogates for asymmetric synthesis [30]. 1,1-Diacetates, on the other hand, are ambient substrates containing two types of reactive carbon centers, the carbon atom of the protected aldehyde function and the carbonyl group in the ester moieties [31]. To the best of our knowledge and in spite of the abovementioned important applicabilities of oximes, semicarbazones and acylals, there is no any report about the preparation of biscoumarins using these types of substrates.
In recent years and because of the unique properties of nanocatalysts, synthetic chemists focused on the preparation and characterization of these types of compounds [32]. Among them nano metal oxides are under considerable attention of many researchers. Nano-ZnO, as a solid acid reagent, is one of these catalysts which have found a wide range of applications in organic transformations [33–41]. One of the most important applicabilities of nano-ZnO is based on its photocatalytic activity which occurs under ultraviolet light excitation [42–46]. In 2008, Ma et al. [47] reported that the catalytic activity of TiO2 in photocatalytic degradation of methyl orange can be highly improved by doping with tungsten. They attributed this result to the help of the doped W in trapping of photogenerated electrons and the enhancement of the surface acidity of TiO2. In 2013 and on the basis of the Ma’s report, Moafi et al. [48] showed that doping of ZnO with 4 mol % of tungsten, in the same manner, can improve the photocatalytic activity of this reagent in photodegradation of methylene blue.
Methods
General
All chemicals were purchased from Merck, Aldrich and Fluka Chemical Companies and used without further purification. Products were characterized by their physical constants and comparison with authentic samples. The purity determination of the substrates and reaction monitoring were accompanied by TLC using silica gel SIL G/UV 254 plates.
To investigate the morphology of the W-doped sample, scanning electron microscopy (SEM) images were obtained on a Philips, XL30. The particles sizes were obtained by transmission electron microscope (TEM) images on a Philips CM10 instrument with an accelerating voltage of 100 kV. Elemental analyses of the samples were carried out by ICP-OES. Measurements were made on an ICP-OES Vista-Pro (Varian), after dissolution of the samples in a HNO3:HF:H2O mixture.
The BET specific surface areas of the synthesized nanocomposite were determined by nitrogen adsorption at liquid nitrogen temperature on a Sibata SA-1100 surface area analyzer. X-ray diffraction measurements were recorded by a Philips PW1840 diffractometer with Cu-Kα radiation, scan rate 0.02 × 2θ/s and within a range of 2θ of 10°–80° at room temperature.
A Perkin Elmer 781 Spectrophotometer was used to record the IR spectra. The 1H NMR spectra were recorded with Bruker Avance 300, 400 and 500 MHz instruments. All chemical shifts are quoted in parts per million (ppm) relative to TMS using deuterated solvent. The 13C NMR data were collected on Bruker Avance 100 MHz instrument. Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.
Catalyst preparation
The W-doped ZnO nanocomposite was prepared by sol–gel method using the precursors of zinc and tungsten. Zinc acetate dihydrate [Zn(Ac)2·2H2O] was used as zinc oxide source. In a typical procedure, 0.02 mol of zinc acetate dihydrate was dissolved in 50 mL of methanol and heated at 50 °C with stirring for half an hour. Then, certain amounts of sodium tungstate (8 mol % with respect to zinc acetate dihydrate) was dissolved in a mixture of water/methanol [10 mL (2:8)] under vigorous stirring and then the solution was added dropwise into the mixture of zinc acetate dihydrate and methanol, thus making precursor solution A. Afterwards, 0.04 mol of sodium hydroxide was dissolved in 50 mL of methanol and heated at 50 °C with stirring for 1 h, making precursor solution B. To make ZnO nano-sol, the solution of sodium hydroxide (solution B) was added dropwise into the solution A under constant stirring for half an hour and then the mixture was heated at 50 °C for further half an hour. Subsequently, a homogenous sol was obtained. The obtained solution was precipitated after continuous stirring for 2 h and cooling at room temperature. After 24 h, the colloidal solution was washed several times with methanol. Finally, so obtained precipitate was dried at 80 °C and then calcinated at 300 °C for 3 h. Using the same procedure, 2, 4, 6.0 mol % W-doped ZnO and undoped ZnO samples were obtained. The obtained results clarified that the sample with 8 mol % W has highest catalytic activity.
General procedure
General procedure for the synthesis of biscoumarins
A mixture of the aldehyde and/or the protected derivative of aldehyde (1 mmol), 4-hydroxycoumarin (2 mmol) and W-ZnO (20 mg) in H2O (3 mL) was stirred at 80 °C for the appropriate time. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature and the solvent was evaporated. Then the solid residue was dissolved in CH2Cl2 (5 mL) and filtered to separate the catalyst. After evaporation of the solvent, the residue was recrystallized from EtOH and water (95:5) to afford the pure product.
Spectral data of the selected products
-
(a)
Biscoumarin derivative of benzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 300 MHz): δ = 6.12 (1H, s), 7.24–8.10 (13H, m), 11.33 (1H, s), 11.56 (1H, s) ppm.
-
(b)
Biscoumarin derivative of 4-chlorobenzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 500 MHz): δ = 6.04 (1H, s), 8.09–7.17 (12H, m), 11.32 (1H, s), 11.54 (1H, s) ppm.
-
(c)
Biscoumarin derivative of 2-chlorobenzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 300 MHz,): δ = 6.14 (1H, s), 8.03–7.22 (12H, m), 10.93 (1H, s), 11.63 (1H, s) ppm.
-
(d)
Biscoumarin derivative of 4-bromobenzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 300 MHz,): δ = 6.01 (1H, s), 7.10–8.06 (12H, m), 11.31 (1H, brs), 11.54 (1H, brs)
-
(e)
Biscoumarin derivative of 4-nitrobenzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 300 MHz,): δ = 6.14 (1H, s), 7.28–8.22 (12H, m), 11.40 (1H, s), 11.59 (1H, s) ppm.
-
(f)
Biscoumarin derivative of 3-nitrobenzaldehyde with 4-hydroxycoumarin: 1H NMR (DMSO-d 6 , 400 MHz): δ = 6.39 (1H, s), 7.28–8.04 (12H, m), 8.04–9.52 (2H, m) ppm.
-
(g)
Biscoumarin derivative of 2-nitrobenzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 300 MHz,): δ = 6.6 (1H, s), 6.72–7.94 (12H, m), 11.24 (2H, brs) ppm.
-
(h)
Biscoumarin derivative of 4-methoxybenzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 300 MHz): δ = 3.80 (3H, s), 6.05 (1H, s), 6.85 (2H, d, J = 8.7 Hz), 7.13 (2H, d, J = 8.7 Hz), 7.30–7.42 (4H, m), 7.63 (2H, t, J = 8.2 Hz), 8.03 (2H, dd, J = 8.4 Hz), 11.29 (1H, brs), 11.51 (1H, brs)
-
(i)
Biscoumarin derivative of 2-hydroxybenzaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 500 MHz): δ = 5.41 (1H, s), 7.10–7.20 (2H, m), 7.23 (2H, d, J = 8.0 Hz), 7.30–7.38 (3H, m), 7.40–7.50 (2H, m), 7.53 (1H, td, J1 = 7.5, J2 = 1.5 Hz), 7.67 (1H, td, J1 = 7.8, J2 = 1.5 Hz), 8.07 (1H, dd, J1 = 7.9, J2 = 1.5 Hz), 8.19 (1H, dd, J1 = 8.0, J2 = 1.5 Hz), 10.43 (1H, s) ppm.
-
(j)
Biscoumarin derivative of 4-(N,N-dimethyl)benzaldehyde with 4-hydroxycoumarin: 1H NMR (DMSO-d 6 , 400 MHz): δ = 3.2 (6H, s), 6.31 (1H, s), 7.23–7.84 (12H, m) ppm.
-
(k)
Biscoumarin derivative of cinnamaldehyde with 4-hydroxycoumarin: 1H NMR (CDCl3, 300 MHz): δ = 4.9 (1H, d, J = 3.5 Hz) 6.85 (1H, d, J = 15.4 Hz), 7.15–7.18 (5H, m), 7.20 (2H, td, J1 = 8.0, J2 = 2.5 Hz), 7.25 (1H, dd, J1 = 15.4, J2 = 3.5 Hz), 7.29 (2H, d, J = 8.0 Hz), 7.51 (2H, td, J1 = 8.0, J2 = 1.7 Hz), 7.89 (2H, d, J = 8.0 Hz) ppm.
-
(l)
Biscoumarin derivative of pyridine-4-carbaldehyde with 4-hydroxycoumarin: 1H NMR (DMSO-d 6 , 400 MHz): δ = 6.49 (s, 1H, s), 7.23 (2H, t, J = 7.2 Hz), 7.28 (2H, d, J = 8.4 Hz), 7.5 (2H, t, J = 7.2 Hz), 7.81–7.83 (4H, m), 8.6 (2H, d, J = 6 Hz), 16.97 (2H, brs) ppm.
-
(m)
Biscoumarin derivative of pyridine-3-carbaldehyde with 4-hydroxycoumarin: 1H NMR (DMSO-d 6 , 400 MHz): δ = 6.43 (1H, s), 7.26 (2H, t, J = 7.6 Hz), 7.3 (2H, d, J = 8 Hz), 7.56 (2H, td, J1 = 7.6 Hz, J2 = 1.2 Hz), 7. 82 (2H, dd, J1 = 7.8 Hz, J2 = 1.2 Hz), 7.94 (1H, td, J1 = 7 Hz, J2 = 2 Hz), 8.37 (1H, d, J = 8 Hz), 8.66 (1H, s), 8.72 (1H, d, J = 5.2 Hz), 16.93 (2H, brs) ppm.
-
(n)
Biscoumarin derivative of 3-phenylpropanal with 4-hydroxycoumarin: 1H NMR (DMSO-d 6 , 400 MHz): δ = 1.89 (2H, m), 2.67 (2H, m), 5.8 (1H, t, J = 6.8 Hz), 6.5–6.8 (5H, m), 7.30 (2H, td, J1 = 8.0, J2 = 2.4 Hz), 7.33 (2H, d, J = 8.0 Hz), 7.65 (2H, td, J1 = 8.0, J2 = 2.2 Hz), 7.88 (2H, d, J1 = 8.0 Hz) ppm.
Results and discussion
Catalyst characterization
Powder X-ray diffraction
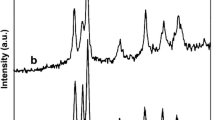
Figure 1 shows the XRD patterns of W-doped ZnO nanocomposite. The sample showed a hexagonal wurtzite crystal structure and high crystallinity of ZnO. The peaks at 2θ = 31.7°, 34.5°, 36.7°, 47.7°, 56.5°, 62.9° and 67.9° are associated with the (100), (002), (101), (102), (110), (103) and (112) planes of the ZnO hexagonal wurtzite structure. The diffraction peaks of the W-ZnO are broad, indicating a small crystal size of this sample. The XRD pattern of the W-ZnO catalyst shows that there is no change in the crystal structure upon tungsten doping process. However, it can be indicated that W+6 ions are uniformly dispersed on ZnO nanoparticles in the form of highly dispersed WO3 clusters.
There were no detectable peaks relating to the existence of a separate dopant metal phase in any corresponding pattern. This could be attributed to the fact that the dopant metals/metal oxides were too low in concentration and/or amorphous structure to be seen as a separate phase. The real W content in W-ZnO sample was measured by ICP-OES (Table 1). The weight ratio of W/Zn in the W-ZnO nanocomposite was 0.228 %.
Surface area and pore distribution measurements
The surface area of W-ZnO nanocomposite, which influences the catalytic activity, was determined using the nitrogen gas adsorption method. The BET surface area of the prepared W-ZnO yielded relatively high surface area (93.70 m2/g). The average grain size are calculated using the Scherrer’s equation based on the full width at half maximum (FWHM) of the (101) peak of the compounds. The results are summarized in Table 5.
SEM analysis
The surface morphology and dispersion of the sample were determined by scanning electron microscopy (SEM). Figure 2 shows SEM micrographs of 8 mol % W-ZnO. The image reveals that the particles in this sample have relatively a sphere-like morphology and the nano particles were composed of agglomerates of fine W-doped ZnO nanoparticles and particle size less than 100 nm. The small and uniform size of the prepared reagent can be affected by its catalytic performance.
TEM analysis
Figure 3 depicts transmission electron micrograph of 8 mol % W-ZnO. The TEM image of W-ZnO shows that the sample consists of fine particles with diameters less than 20 nm in size.
Catalytic activity
In recent years, preparation and use of nanocatalysts in organic transformations became an important part of our ongoing research program [32, 49–51]. In continuation of these studies, and on the basis of the Fallah Moafi’s report [48], we anticipated that W-doped ZnO nanocomposite can be used as an efficient solid acid catalyst for the acceleration of the reactions which need the use of an acidic catalyst to speed up. So we were interested to investigate the applicability of this reagent in the promotion of the synthesis of biscoumarins.
Our initial studies clarified that to obtain the best results the amounts of tungsten should be enhanced to 8 mol %. So we have prepared, identified and studied the role of the 8 mol % W-doped ZnO nanocomposite in the synthesis of biscoumarins.
At the first step and to optimize the reaction conditions, the prepared catalyst was used for the promotion of the condensation of 4-chlorobenzaldehyde with 4-hydroxycoumarin and compared the effect of different solvents and solvent-free conditions and also the effect of the catalyst load on the reaction yield and time at thermal conditions. The results are shown in Table 2. On the basis of these results it can be concluded that the best results can be obtained under the conditions shown in Scheme 1. It is interesting to note that using lower amounts of the catalyst (Table 2, entry 2) and/or lower amounts of W in the preparation of the catalyst (Table 2, entries 3, 4) resulted the products’ longer reaction times and/or lower yields.
To study the efficiency of W-ZnO in the preparation of biscoumarin derivatives, a wide range of aromatic, aliphatic and heterocyclic aldehydes were reacted with 4-hydroxycoumarin under the optimal reaction conditions and the obtained results are tabulated in Table 3.
It was observed that under the selected conditions, aromatic aldehydes containing electron-withdrawing groups as well as electron-donating groups were easily reacted in short reaction times with good to excellent isolated yields (Table 3, entries 1–12). α,β-Unsaturated aldehydes were also reacted in high yields using this procedure without the formation of any by-products (Table 3, entry 13).
After the abovementioned studies and for the first time, we have studied the applicability of the same method in the preparation of biscoumarins from the protected aldehydes (e.g., oximes, semicarbazones and 1,1-diacetates). The obtained results showed that under the same reaction conditions, the protected derivatives of aldehydes were efficiently converted to the requested biscoumarins during short reaction times in high yields (Table 4).
A plausible mechanism of the reaction is shown in Scheme 2 [60].
To show the efficiency of the present method, we have compared our results obtained from the synthesis of biscoumarins catalyzed by W-ZnO with some of the other results reported in the literature. As it can be seen in Table 5, some of the previously reported methods are performed in the presence of toxic reagents (Table 5, entry 2) or solvents (Table 5, entries 4–6). It should be noted that in the absence of the catalyst, the reaction is completed during much longer times (Table 5, entry 1).
Conclusion
In summary, we have introduced W-doped ZnO nanocomposite as a highly efficient nanocatalyst for the acceleration of the synthesis of biscoumarins under mild and completely heterogeneous reaction conditions. This method has several advantages such as ease of preparation and handling of the catalyst, easy work-up procedure, high reaction rates and excellent yields. Also, and for the first time different types of protected aldehydes were successfully employed in these types of reactions and the corresponding products were obtained in high to excellent yields.
References
Jung, J.C., Park, O.S.: Synthetic approaches and biological activities of 4-hydroxycoumarin derivatives. Molecules 14, 4790–4803 (2009)
Su, C.X., Mouscadet, J.F., Chiang, C.C., Tsai, H.J., Hsu, L.Y.: Integrase inhibition of biscoumarin analogues. Chem. Pharm. Bull. 54, 682–686 (2006)
Kancheva, V.D., Boranova, V.P., Nechev, J., Manolov, I.I.: Structure-activity relationships of new 4-hydroxy bis-coumarins as radical scavengers and chain-breaking antioxidants. Biochimie 92, 1138–1146 (2010)
Khan, K.M., Iqbal, S., Lodhi, M.A., Maharvi, G.M., Ullah, Z., Choudhary, M.I., Rahman, A., Perveen, S.: Biscoumarin: new class of urease inhibitors; economical synthesis and activity. Bioorg. Med. Chem. 12, 1963–1968 (2004)
Kostova, I., Momekov, G., Zaharieva, M., Karaivanova, M.: Cytotoxic activity of new lanthanum (III complexes of bis-coumarins. Eur. J. Med. Chem. 40, 542–551 (2005)
Gupta, A.D., Samanta, S., Mondal, R., Mallika, A.K.: A Convenient, eco-friendly, and efficient method for synthesis of 3,3′-arylmethylene-bis-4-hydroxycoumarins “on water”. Bull. Korean. Chem. Soc. 33, 4239–4242 (2012)
Karimi-Jaberi, Z., Nazarifar, M.R., Pooladian, B.: Tris(hydrogensulfato) boron as a solid heterogeneous catalyst for the rapid synthesis of α,α′-benzylidenebis(4-hydroxycoumarin) derivatives. Chin. Chem. Lett. 23, 781–784 (2012)
Padalkar, V., Phatangare, K., Takale, S., Pisal, R., Chaskar, A.: Silica supported sodium hydrogen sulfate and indion 190 resin: an efficient and heterogeneous catalyst for facile synthesis of bis-(4-hydroxycoumarin-3-yl)methanes. J. Saudi. Chem. Soc. (2012). doi:10.1016/j.jscs.2011.12.015
Khurana, J.M., Kumar, S.: Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett. 50, 4125–4127 (2009)
Heravi, M.M., Sadjadi, S., Mokhtari, H.N., Oskooie, H., Bamoharram, F.F.: Role of various heteropolyacids in the reaction of 4-hydroxycoumarin, aldehydes and ethylcyanoacetate. Catal. Commun. 10, 1643–1646 (2009)
Singh, P., Kumar, P., Katyal, A., Kalra, R., Dass, S.K., Prakash, S., Chandra, R.: Phosphotungstic acid: an efficient catalyst for the aqueous phase synthesis of bis-(4-hydroxycoumarin-3-yl)methanes. Catal. Lett. 134, 303–308 (2010)
Mehrabi, H., Abusaidi, H.: Synthesis of biscoumarin and 3,4-dihydro pyrano[c]chromene derivatives catalysed by sodium dodecyl sulfate (SDS) in neat water. J. Iran Chem. Soc. 7, 890–894 (2010)
ParvanakBoroujeni, K., Ghasemi, P.: Synthesis and application of a novel strong and stable supported ionic liquid catalyst with both Lewis and Brønsted acid sites. Catal. Commun. 37, 50–54 (2013)
Shirini, F., Abedini, M., AbroonKiaroudi, S.: Introduction of titania sulfonic acid [TiO2–SO3H] as a new, efficient and reusable heterogenous solid acid catalyst for the synthesis of biscoumarins. Phosphorus Sulfur Silicon 189, 1279–1288 (2014)
Greene, T.W., Wuts, P.G.M.: Protective Groups in Organic Synthesis, 3rd edn. Wiley, New York (1991)
Curini, M., Rostai, O., Pisani, E., Costantino U.: Heterogeneous catalysis in carbonyl regeneration from oximes, semicarbazones, and tosylhydrazones by zirconium sulphophenyl phosphonate. Synlett 4, 333–334 (1996)
Kabalka, G.W., Pace, R.D., Wadgaonkar, P.P.,: Oximes from conjugated nitroalkenes using Pd/C-ammonium formate CTH. Synth. Commun. 20, 2453–2458 (19990)
Fujisawa, T., Kurita, Y., Sato, T.: A convenient synthesis of ketoximes from grignard reagents and nitro compounds activated by N,N-dimethyl chloromethyleniminium chloride. Chem. Lett. 10, 1537–1540 (1983)
Barton, D.H.R., Beaton, J.M., Geller, L.E., Pechet, M.M.,: A new photochemical reaction. J. Am. Chem. Soc. 83, 4076–4083 (1961)
Lee, K. Han, S.B., Yoo, E.M., Chung, S.R., Oh, H., Hong, S.: Efficient transformation of aldoximes to nitriles using 2-chloro-1-methylpyridinium iodide under mild conditions. Synth. Commun. 34, 1775–1782 (2004)
Sarvari, M.S.: ZnO/ CH3COCl: a new and highly efficient catalyst for dehydration of aldoximes into nitriles under solvent-free condition. Synthesis 5, 787–790 (2005)
Movassagh, B., Fazeli, A.: Direct synthesis of aromatic nitriles from aldehydes using hydroxylamine and oxalyl chloride. Synth. Commun. 37,623–628 (2007)
Schoenewalat, E.F., Kinnel, R.B., Davis, P.: Improved synthesis of anti-benzaldoxime. Concomitant cleavage and formylation of nitrones. J. Org. Chem. 33, 4270–4272 (1968)
Abiraj, K., Channe, G.D.: Zinc/ammonium formate: a new facile system for the rapid and selective reduction of oximes to amines. J. Chem. Res. (S) 6, 332–334 (2003)
Ghiaci, M., Hassan, I.G.: A facile beckmann rearrangement of oximes with AlCl3 in the solid state. Synth. Commun. 28, 2275–2280 (1998)
Peter, W., Joan, M.F., Laura, S.: Microwave promoted oxazole synthesis: cyclocondensation cascade of oximes and acyl chlorides. Tetrahedron Lett. 46, 5463–5466 (2005)
Hajipour, A.R., Mahboubghah, N.J.: 1-Benzyl-4-aza-1-azoniabicyclo [2.2.2]octaneperiodate: a mild and efficient oxidant for the cleavage of oxime double bonds under anhydrous conditions. J. Chem. Res. (S) 122–123 (1998)
Gregory, M.J.: Evidence for a cyclic AA11 mechanism in the hydrolysis of benzylidenediacetates. J. Chem. Soc. B 1201–1207 (1970)
Heerden, F.R., Huyser, J.J., Williams, D.B.G., Holzapfer, C.W.: Palladium-catalysed substitution reactions of germinal allylicdiacetates. Tetrahedron Lett. 39, 5281–5284 (1998)
Trost, B.M., Lee, C.: gem-Diacetates as carbonyl surrogates for asymmetric synthesis. Total syntheses of sphingofungins E and F. J. Am. Chem. Soc. 123, 12191–12201 (2001)
Sandberg, M. Sydnes, L.K.: The chemistry of acylals. Part II. Formation of nitriles by treatment of acylals with trimethylsilylazide in the presence of a Lewis acid. Tetrahedron. Lett. 39, 6361–6364 (1998)
Shirini, F., Abedini, M.: Application of nanocatalysts in multi-component reactions. J. Nanosci. Nanotech. 13, 4838–4860 (2013)
Safaei-Ghomi, J., Ghasemzadeh, M.A.: Zinc oxide nanoparticles: A highly efficient and readily recyclable catalyst for the synthesis of xanthenes. Chin. Chem. Lett. 23, 1225–1229 (2012)
Maghsoodlou, M.T., Habibi-Khorassani, S.M., Shahkarami, Z., Maleki, N., Rostamizadeh, M.: An efficient synthesis of 2,2′-arylmethylene bis (3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) and 1,8-dioxo-octahydroxanthenes using ZnO and ZnO–acetyl chloride. Chin. Chem. Lett. 21, 686–689 (2010)
Tamaddon, F., Sabeti, M.R., Jafari, A.A., Tirgir, F., Keshavarz, E.: ZnO and ZnO-nanoparticles: Efficient and reusable heterogeneous catalysts for one-pot synthesis of N-acylsulfonamides and sulfonate esters. J. Mol. Catal. A Chem. 351, 41–45 (2011)
Paul, S., Bhattacharyya, P., Das, A.R.: One-pot synthesis of dihydropyrano[2,3-c]chromenes via a three component coupling of aromatic aldehydes, malononitrile, and 3-hydroxycoumarin catalyzed by nano-structured ZnO in water: a green protocol. Tetrahedron Lett. 52, 4636–4641 (2011)
Ghosh, P.P., Das, A.R.: Nano crystalline ZnO: a competent and reusable catalyst for one pot synthesis of novel benzylaminocoumarin derivatives in aqueous media. Tetrahedron Lett. 53, 3140–3143 (2012)
Bhattacharyya, P., Pradhan, K., Paul, S., Das, A.R.: Nano crystalline ZnO catalyzed one pot multicomponent reaction for an easy access of fully decorated 4H-pyran scaffolds and its rearrangement to 2-pyridone nucleus in aqueous media. Tetrahedron Lett. 53, 4687–4691 (2012)
Kassaee, M.Z., Movahedi, F., Masrouri, H.: ZnO nanoparticles as an efficient catalyst for the one-pot synthesis of α-amino phosphonates. Synlett 8, 1326–1330 (2009)
Dharma Rao, G.B., Kaushik, M.P., Halve, A.K.: An efficient synthesis of naphtha[1,2-e]oxazinone and 14-substituted-14H-dibenzo[a, j]xanthene derivatives promoted by zinc oxide nanoparticle under thermal and solvent-free conditions. Tetrahedron Lett. 53, 2741–2744 (2012)
Kassaee, M.Z., Masrouri, H., Movahedi, F.: ZnO-nanoparticlepromoted synthesis of polyhydroquinoline derivatives via multicomponent Hantzsch reaction. Monatsh. Chem. 141, 317–322 (2010)
Sun, J.H., Dong, S.Y., Feng, J.L., Yin, X.J., Zhao, X.C.: Enhanced sunlight photocatalytic performance of Sn-doped ZnO for Methylene Blue degradation. J. Mol. Catal. A: Chem. 335, 145–150 (2011)
Patil, A.B., Patil, K.R., Pardeshi, S.K.: Ecofriendly synthesis and solar photocatalytic activity of S-doped ZnO. J. Hazard. Mater. 183, 315–323 (2010)
Wu, C., Shen, L., Yu, H., Huang, Q., Zhang, Y.C.: Synthesis of Sn-doped ZnO nanorods and their photocatalytic properties. Mater. Res. Bull. 46, 1107–1112 (2011)
Karunakaran, C., Gomathisankar, P., Manikandan, G.: Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater. Chem. Phys. 123, 585–594 (2010)
Barick, K. C., Singh, S., Aslamb, M., Bahadur, D.: Porosity and photocatalytic studies of transition metal doped ZnO nanoclusters. Microporous. Mesoporous. Mater. 134, 195–202 (2010)
Tian, H., Ma, J., Li, K., Li, J.: Photocatalytic degradation of methyl orange with W-doped TiO2 synthesized by a hydrothermal method. Mater. Chem. Phys. 112, 47–51 (2008)
Fallah Moafi, H., Zanjanchi, M.A., Fallah Shojaie, A.: Tungsten-doped ZnO nanocomposite: Synthesis, characterization, and highly active photocatalyst toward dye photodegradation. Mater. Chem. Phys. 139, 856–864 (2013)
Shirini, F., Mamaghani, M., Atghia, S.V.: Sulfonic acid-functionalized ordered nanoporous Na+-montmorillonite (SANM): A novel, efficient and recyclable catalyst for the chemoselective N-Boc protection of amines in solvent less media. Catal. Commun. 12, 1088–1094 (2011)
Shirini, F., Atghia, S.V., Ghazi Jirdehi, M.: Nanocrystalline TiO2–HClO4 as a new, efficient and recyclable catalyst for the chemoselectivetrimethylsilylation of alcohols, phenols and deprotection of silyl ethers. Catal. Commun. 18, 5–10 (2012)
Shirini, F., Akbari-Dadamahaleh, S., Mohammad-Khah, A.: Rice-husk-supported FeCl3 nano-particles: Introduction of a mild, efficient and reusable catalyst for some of the multi-component reactions. C. R. Chimie. 16, 945–955 (2013)
Tavakoli-Hoseini, N., Heravi, M.M., Bamoharram, F.F., Davoodnia, A., Ghassemzadeh, M.: An unexpected tetracyclic product isolated during the synthesis of biscoumarins catalyzed by [MIM(CH2)4SO3H][HSO4]: Characterization and X-ray crystal structure of 7-(2-hydroxy-4-oxo-4H-chromen-3-yl)-6H,7H-chromeno[4,3-b]chromen-6-one. J. Mol. Liq. 163, 122–127 (2011)
Khurana, J.M., Vij, K.: Nickel nanoparticles: A highly efficient catalyst for one pot synthesis of tetraketones and biscoumarins. J. Chem. Sci. 124, 907–912 (2012)
Al-Kadasi, A.M.A., Nazeruddin, G.M.: Ultrasound assisted catalyst-free one-pot synthesis of biscoumarins in neat water. Int. J. Chem. Sci. 10, 324–330 (2012)
Niknam, K., Jamali, A.: Silica-bonded N-propylpiperazine sodium n-propionate as recyclable basic catalyst for synthesis of 3,4-dihydropyrano[c]chromene derivatives and biscoumarins. Chin. J. Catal. 33, 319–326 (2012)
Manolov, I., Maichle-Moessmer, C., Nicolova, I., Danchev, N.: Synthesis and anticoagulant activities of substituted 2,4-diketochromans, biscoumarins, and chromanocoumarins. Arch. Pharm. Chem. Life. Sci. 339, 319–326 (2006)
Manolov, I., Maichle-Moessmer, C., Danchev, N.: Toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 41, 882–890 (2006)
Seddighi, M., Shirini, F., Mamaghani, M.: Sulfonated rice husk ash (RHA-SO3H) as a highly efficient and reusable catalyst for the synthesis of some of bisheterocyclic compounds. RSC Adv. 3, 24046–24053 (2013)
Kidwai, M., Bansal, V., Mothsra, P., Saxena, S., Somvanshi, R.K., Dey, S., Singh, T.P.: Molecular iodine: a versatile catalyst for the synthesis of bis(4-hydroxycoumarin) methanes in water. J. Mol. Catal. A. 265, 76–81 (2007)
Tabatabaeian, K., Heidari, H., Khorshidi, A.R., Mamaghani, M., Mahmoodi, N.O.: Synthesis of biscoumarin derivatives by the reaction of aldehydes and 4-hydroxycoumarin using ruthenium(III) chloride hydrate as a versatile homogeneous catalyst. J. Serb. Chem. Soc. 77, 407–413 (2012)
Acknowledgments
We are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Shirini, F., Abedini, M., Zamani, S. et al. Introduction of W-doped ZnO nanocomposite as a new and efficient nanocatalyst for the synthesis of biscoumarins in water. J Nanostruct Chem 5, 123–130 (2015). https://doi.org/10.1007/s40097-014-0143-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-014-0143-9