Abstract
A new study for a boron neutron capture therapy irradiation facility, based on a 2.5 MeV proton accelerator on a thick Li target as neutron converter, is presented here. The beam shaping assembly (BSA) modeling has been performed with the use of the MCNP5 Monte Carlo code. The fast (i.e., > 10 keV) neutron component yielded by the 7Li(p,n)7Be reaction is slowed down through TiF3 neutron spectrum shifter, while to obtain a high-quality epithermal neutron beam at the beam port exit additional layers for thermal neutrons removal and shielding of gamma rays were used. Moreover, 60Ni and Ti6Al14V were selected to filter out and further remove the residual fast neutron component, while cadmium was chosen as thermal neutrons absorber, and bismuth was selected for gamma rays shielding. The therapeutic effectiveness of the proposed BSA was evaluated by performing a set of dose-equivalent distribution calculations in a standard Snyder head phantom. The simulation results show that the proposed BSA modeling meets all the recommended by IAEA criteria and provides one possible technical choice for an accelerator-based BNCT irradiation facility in a hospital environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neutrons can easily diffuse inside matter before interacting, due to the null electric charge. Because of such a unique property, such particles are widely used as probes in many research fields, such as geology, biology [1], engineering [2] and for security applications as well. In medicine, one promising radiotherapy method which is based on neutrons is the boron neutron capture therapy (BNCT).
BNCT, however, is not a new method, since the first BNCT treatment performed in 1936 on a glioma patient [3]. Despite the progress achieved so far, there are types of cancers, e.g., the glioblastoma multiforme, recurrent cancers of the head, neck and liver where the conventional treatment methods, such as surgery, chemotherapy and radiotherapy have revealed to be less effective. For these types of cancers, BNCT might represent an interesting alternative therapeutic approach. BNCT is a binary treatment modality: First a 10B carrier drug is delivered into the patient body toward the final tumor tissue target and when the ratio of 10B atoms concentration between the cancer cells and the surrounding healthy tissues is large enough (i.e., usually ~ 3–4 for the most common BSA/BSH compounds), the patient is irradiated with a neutron beam inducing the 10B(n,α)7Li nuclear reaction route with thermal neutrons.
Both the range of the α-particles and 7Li break-up fragments into neighboring tissues are very short (~ 5–8 microns at the average); therefore, the highly ionising radiation damage is nearly only the original 10B-containing tumors cells.
Thermal neutrons are suitable for surface or at least shallow tumors treatment only, because of their short mean free path in soft tissues. Usually, cancer cells are located deeper inside the human body, and in these cases epithermal neutrons with an energy range between 1 eV and 10 keV reveal more effective. The patient trials for deep-seated tumors, conducted all over the world in the last 20–25 years, have been performed making use of just 7–8 reactor-based epithermal neutron BNCT facilities. High-flux epithermal neutron beams with low fast neutron and gamma contamination are required, fulfilling the preliminary IAEA criteria [4]. To date, the existing BNCT facilities are based on nuclear reactors, but the scientific community has prompted R&D activities for alternative projects which use accelerator-driven neutron sources [5,6,7,8,9,10,11,12,13,14]. In present work, a beam shaping assembly (BSA) optimization study based on a 2.5 MeV proton accelerator and making use of a thick Li target is presented, which fulfills all the recommended IAEA criteria as preliminary in-air figure of merit (FOM) parameters. Main results from such a study are then compared with other published BSA facilities which are based on similar accelerator-based neutron sources. In such a study, we have taken into account neutrons produced via the 7Li(p,n)7Be reactions by a 2.5 MeV, 10 mA proton beam into a thick lithium target. All the required simulations for such a proposed BNCT facility modeling have at last been performed with the MCNP5 Monte Carlo transport code [15].
Materials and methods
Neutron source
Different neutron sources have been investigated for the modeling of a BNCT irradiation facility. Nuclear reactors represent the common choice for high-intensity beams despite their drawbacks (expensive, sizeable and with different safety concerns) because usually no other high-intensity neutron source is suitable for such a purpose. On the other hand, about Sealed Tube (ST) generators, based on either DD or DT fusion nuclear reaction, although technical improvements have been obtained in the last 20 years, still continues to have low intensity for BNCT treatment [5, 6, 16, 17].
Studies on protons, deuterons or electron based accelerators have also been assessed such as capable to provide neutron beam fluxes comparable to that provided by nuclear reactors. In this work, a Monte Carlo modeling study on a BSA irradiation system, driven by a Rf focused interdigital (RFI) linac structure, has been carried out. Based on the previous work by Bayanov et al. [18], Fantidis [14] and Lee [19] the expected neutron source level has been estimated to be 8.83E+12 s−1 by using 10 mA, 2.5 MeV protons and supposing a water-cooled thick lithium target. The related prompt gamma ray spectrum which is also produced may be found from previous works by Kiss et al. [20] and Lee et al. [21].
BSA modeling
In order to ensure the requested neutron beam parameters at the beam exit port, a proper spectrum shifter system, or BSA, is necessary. The BSA includes mainly four parts, namely the epithermal spectrum shifter, the reflector for epithermal neutrons and some absorbers/beam delimiter for low energy (i.e., thermal) neutrons and shielding for gamma radiation produced both in the neutron converter and during the neutron beam tailoring. The neutron spectrum shifter, the core of the system, has to slow down the fast neutrons yielded by the source, i.e., having energy larger than 10 keV, in a selected way without increasing the fraction of thermal neutrons in order to get a net accumulation in the epithermal energy range (i.e., 1 eV–10 keV). A reflector has to be included to either limit the neutron losses or scatter neutrons toward the beam port, while further improving the quality of the beam.
Dose evaluation
In order to evaluate the therapeutic effectiveness of each BNCT BSA modeling configuration considered, some in-air figures of merit (FOM) parameters are taken as reference (see Table 1) and widely used as a preliminary assessment. In addition, the Snyder head phantom calculations about profiles determination of main dose parameters and their related effectiveness were performed [14, 22]. These parameters include the advantage depth (AD), the advantage depth dose rate (ADDR), the treatment time (TT), the therapeutic depth (TD) and the advantage ratio (AR). AD is defined as the depth in tissue at which a tumor receives a dose equal to the maximum dose delivered at the healthy tissue. ADDR expresses the maximum delivered dose-equivalent rate to healthy tissue. TT is the required treatment time needed to impart the maximum dose to the healthy tissue while keeping below the limit of 12.5 relative biological effectiveness (RBE) Gy [8]. TD is the depth at which the cancer dose falls below twice the maximum dose to healthy tissue, and AR is defined as the ratio between of the total therapeutic dose that would be delivered to tumor tissue and the integral dose delivered to normal tissue at the AD [23].
The total absorbed tissue dose equivalent (DT) can be obtained by the following equation:
where wγ, wfast, wN and wB are the weighting factors, or RBE values, for γ rays, fast neutrons, (n,p) reaction on nitrogen and radiative capture (n, gamma) reactions for boron atoms which accounts for the neutron thermal component, respectively, with values wγ = 1, wfast = 3.2, wN = 3.2 and wB = 1.3 for boron in tissue and 3.8 for boron in the tumor [23, 24]. Dγ, Dfast, DN and DB are the calculated dose components from γ rays, fast neutrons, nitrogen and boron correspondingly. In the calculations performed, the 10B concentration in the tumor is usually set at 40 ppm and a 4:1 ratio of 10B nuclei concentration (distribution) in tumor to healthy tissue was considered [23].
Results and discussion
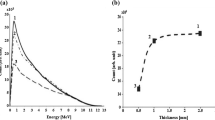
The source spectrum (i.e., number and energy of neutrons) available from the Li thick target is strictly related to the yielded angle with respect to the proton beam line. As known, smaller yielded angles downstream the target allow for having higher neutron fluxes, although with a harder spectrum. For the purposes of this work, in order to achieve a good compromise between an acceptable neutron flux level and a needed epithermal-energy-peaked spectrum distribution, the selected yielded angle chosen was at last 45°. The corresponding neutron source spectrum at this angle is shown in Fig. 1.
The calculated thick-target 7Li(p,n)7Be neutron source spectrum yielded at 45° downstream the target with regard to the incident proton energy of 2.5 MeV (the spectrum based on the data from Ref. [14])
A number of spectrum shifter/reflector/absorber materials and their combinations were simulated with the aim to maximize the epithermal neutron flux and remove all the other beam contaminations at the exit port. The BSA geometrical configuration was studied in a cylindrical shape, and the final model is shown in Fig. 2. Eight different spectrum shifter materials, namely Fluental, MgF2, Al2O3, AlF3, TiF3, Teflon (CF2), CaF2 and 7LiF were considered. Lead (Pb) was selected as reflector, while a combination of TiF3 and an additional material layer has turned out to have the best results as spectrum shifter. A layer to filter out the spectrum harder component can further decrease the presence of the unwanted fast neutrons in the beam. In addition, bismuth (Bi) was selected as gamma ray shielding material, cadmium (Cd) as thermal neutron absorber and lithiated polyethylene (poly-Li) was chosen as delimiter to manage the size of the beam aperture.
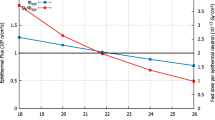
Table 2 reports the in-air parameters for the two optimum BSA configurations investigated. The configuration A includes 14 cm TiF3 spectrum shifter, 30 cm 60Ni fast neutron filter, 3.5 cm Bi gamma ray shielding and 0.1 cm Cd thermal neutron absorber. In the second configuration Ti6Al14V was selected instead of the pure 60Ni due to the higher cost of the highly enriched material: It consists of 15 cm TiF3 spectrum shifter, 27.5 cm Ti6Al14V fast neutron filter, 7 cm Bi gamma ray shielding and 0.1 cm Cd thermal neutron absorber. All the parameters have been calculated using the F2 (surface tally) and FM2 tallies in MCNP5, while for dose distribution calculations being necessary the use of the DE, DF cards. In order to keep the relative errors in all cases lower than 1.5%, cutoff (NPS) values up to 4 × 108 histories were considered. From data reported, it could be concluded that both of the proposed configurations meet all the suggested by IAEA in-air FOM criteria; however, the latter BSA configuration considered is able to provide an epithermal neutron flux level which is almost the double. The neutron spectra calculated at the BSA beam port exit are shown in Fig. 3, while the profiles for fast, epithermal and thermal neutrons flux components expected at the beam port of the suggested BSA configurations are plotted in Fig. 4.
The head phantom which was simulated in this work is derived by the MCNP5 samples files and consists of three ellipsoids for scalp, scull bone and brain. Both the dimensions and the material composition for an adult head proceed from ICRU46 reference parameters [22]. In order to evaluate the proposed BSA configurations here discussed, the MCNP5 calculation results are compared with other published BSA facilities which are based on the similar 2.5 MeV proton beam and a thick lithium target. The results are shown in the next Table 3 where the unacceptable values are noted with italic letters. According to this table, the proposed BNCT facility modeling has similar results with the one discussed in the paper by Montagnini et al. although it is expected to use a proton beam with the half current.
Figure 5 shows the depth–dose distributions calculated in the Snyder’s head phantom for the tumor and healthy tissues. The dose profiles distribution inside the phantom were performed using point detectors (F5 tally) for up to NPS = 8 × 108 histories yielding an accuracy < 2%. Table 4 reports the in-phantom calculated parameters for the proposed accelerator-based BSA for a possible BNCT facility compared to some previous published data for both accelerator-based BSA configurations studied and available (reactor-based) ones. From these results, it may be inferred that both proposed BSA configurations A and B have neutrons beams main parameters which could be suitable for deep tumor treatment into the brain; the AD values are approximately 11.4 and 12.4 cm.
Conclusions
A BSA modeling based on a proton accelerator and a Li thick target has been studied through MCNP5 calculations as a possible, accelerator-based spectrum shifter system for a possible BNCT irradiation facility. According to all calculations carried out, the modeled configurations meet the preliminary by IAEA in-air FOM parameters. Two of them were proposed, and the therapeutic effectiveness for each one was calculated with the aim to get a preliminary dosimetric evaluation in a simulated standard Snyder head phantom. The results obtained with present study, compared to others based on 2.5 MeV proton accelerators and making use of a thick Li target, show that it is possible to further improve the main neutron beam parameters at beam port exit for a possible BNCT facility. The AD values for both configurations have the higher values compared with the other facilities, rendering that the simulated units are suitable for deep-seated tumors in brain.
References
Nicolaou, G., Pietra, R., Sabbioni, E., Mosconi, G., Cassina, G., Seghizzi, P.: Multielement determination of metals in biological specimens of hard metal workers: a study carried out by neutron activation analysis. J. Trace Elem. Electrolytes Health Dis. 1(2), 73–77 (1987)
Bergaoui, K., Reguigui, N., Gary, C.K., Cremer, J.T., Vainionpa, J.H., Piestrup, M.A.: Design, testing and optimization of a neutron radiography system based on a Deuterium–Deuterium (D–D) neutron generator. J. Radioanal. Nucl. Chem. 299(1), 41–51 (2014)
Locher, G.L.: Biological effects and therapeutic possibilities of neutrons. Am. Roentgenol. 36, 1–13 (1936)
IAEA-TECDOC-1223: Current Status of Neutron Capture Therapy. International Atomic Energy Agency, Vienna (2001)
Fantidis, J.G., Antoniadis, A.: Optimization study for BNCT facility based on a DT neutron generator. Int. J. Radiat. Res. 13(1), 13–24 (2015)
Fantidis, J.G., Saitioti, E., Bandekas, D.V., Vordos, N.: Optimised BNCT facility based on a compact DD neutron generator. Int. J. Radiat. Res. 11(4), 207–214 (2013)
Elshahat, B.A., Naqvi, A.A., Maalej, N., et al.: Design calculations of an accelerator based BSA for BNCT of brain cancer. J. Radioanal. Nucl. Chem. 274(3), 539–544 (2007)
Kim, K.O., Kim, J.K., Kim, S.Y.: Optimized therapeutic neutron beam for accelerator-based BNCT by analyzing the neutron angular distribution from 7Li(p, n)7Be reaction. Appl. Radiat. Isot. 67, 1173–1179 (2009)
Montagnini, B., Cerullo, N., Esposito, J., et al.: Spectrum shaping of accelerator-based neutron beams for BNCT. Nucl. Instrum. Method Phys. Res. A 476, 90–98 (2002)
Fantidis, J.G., Nicolaou, G.: Optimization of beam shaping assembly design for boron neutron capture therapy based on a transportable proton accelerator. Alex. Eng. J. (2017). https://doi.org/10.1016/j.aej.2017.08.004
Kim, K.O., Jung, S.H., Kim, S.Y., Kim, G.D., Kim, J.K.: Performance evaluation of a beam-shaping assembly for accelerated-BNCT using the neutron generation experiment of 7Li(p, n)7Be reaction. J. Nucl. Sci. Technol. 45(sup5), 143–146 (2008)
Saito, T., Katabuchi, T., Hales, B., Igashira, M.: Measurement of thick-target gamma-ray production yields of the 7Li(p, p′)7Li and 7Li(p, γ)8Be reactions in the near-threshold energy region for the 7Li(p, n)7Be reaction. J. Nucl. Sci. Technol. 54(2), 253–259 (2017)
Guan, X., Murata, I., Wang, T.: Monte Carlo optimization of an epithermal neutron flux monitor for BNCT. J. Nucl. Sci. Technol. 54(10), 1118–1122 (2017)
Fantidis, J.G.: A study of a transportable thermal neutron radiography unit based on a compact RFI linac. J. Radioanal. Nucl. Chem. 293(1), 95–101 (2012)
Team Monte Carlo: MCNP—a General Monte Carlo N-Particle Transport Code, version 5, Book MCNP (2003)
Cerullo, N., Esposito, J., Leung, K.N., Custodero, S.: An irradiation facility for BNCT application based on a RF-driven D–T neutron source and a new beam shaping assembly. Rev. Sci. Instrum. 73(10), 3614–3618 (2002)
Cerullo, N., Esposito, J., Daquino, G.G.: Spectrum shaping assessment of accelerator-based fusion neutron sources to be used in BNCT treatment. Nucl. Instrum. Methods Phys. Res. Sect. B 213, 641–645 (2004)
Bayanov, B., Belov, V., Kindyuk, V., Oparin, E., Taskaev, S.L.: Lithium neutron producing target for BINP accelerator-based neutron source. Appl. Radiat. Isot. 61(5), 817–821 (2004)
Lee, D.J., Han, C.Y., Park, S.H., Kim, J.K.: An accelerator-based epithermal neutron beam design for BNCT and dosimetric evaluation using a voxel head phantom. Radiat. Prot. Dosim. 110(1–4), 655–660 (2004)
Kiss, A.Z., Koltay, E., Nyako, B., Somorjai, E., Anttila, A., Räisänen, J.: Measurements of relative thick target yields for PIGE analysis on light elements in the proton energy interval 2.4–4.2 MeV. J. Radioanal. Nucl. Chem. 89(1), 123–141 (1985)
Lee, C.L., Zhou, X.-L., Kudchadker, R.J., Harmon, F., Harker, Y.D.: A Monte Carlo dosimetry-based evaluation of the reaction near threshold for accelerator boron neutron capture therapy. Med. Phys. 27(1), 192–202 (2000)
ICRU46: International Commission on Radiation Units and Measurements (1992)
Koivunoro, H., Bleuel, D.L., Nastasi, U., et al.: BNCT dose distribution in liver with epithermal D–D and D–T fusion-based neutron beams. Appl. Radiat. Isot. 61(5), 853–859 (2004)
Rossi, F., et al.: BNCT: neutron dose evaluation using a Monte Carlo code. Radiat. Eff. Defects Solids 164, 350–356 (2009)
Liu, et al.: Renovation of epithermal neutron beam for BNCT at THOR. Appl. Radiat. Isot. 61, 1039–1043 (2004)
Kononov, et al.: Optimization of an accelerator-based epithermal neutron source for neutron capture therapy. Appl. Radiat. Isot. 61, 1009–1013 (2004)
Binns, et al.: Improved dose targeting for a clinical epithermal neutron capture beam using optional 6Li filtration. Int. J. Radiat. Oncol. Biol. Phys. 67(5), 1484–1491 (2007)
Calzetta, L., et al.: BNCT facility at the RA-6 reactor, Current Status of Neutron Capture Therapy. IAEA-TECDOC-1223, VIENNA (2001)
Rasouli, F.S., Masoudi, S.F., Kasesaz, Y.: Design of a model for BSA to meet free beam parameters for BNCT based on multiplier system for D–T neutron source. Ann. Nucl. Energy 39, 18–25 (2012)
Rasouli, F.S., Masoudi, S.F.: Design and optimization of a beam shaping assembly for BNCT based on D–T neutron generator and dose evaluation using a simulated head phantom. Appl. Radiat. Isot. 70, 2755–2762 (2012)
Rasouli, F.S., Masoudi, S.F.: Simulation of the BNCT of brain tumors using MCNP code: beam designing and dose evaluation. Iran. J. Med. Phys. 9(3), 183–192 (2012)
Acknowledgments
Special thanks to Dr. G. E. Nicolaou associate professor in Democritus University of Thrace for valuable support in MCNP calculations and to anonymous reviewer for his valuable contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fantidis, J.G. Beam shaping assembly study for BNCT facility based on a 2.5 MeV proton accelerator on Li target. J Theor Appl Phys 12, 249–256 (2018). https://doi.org/10.1007/s40094-018-0312-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-018-0312-1