Abstract
Purpose
The long-term use of manure as a source of nutrients can promote phosphorus (P) leaching, especially in sandy soils. The aim was to evaluate P mobility from organic and mineral sources in columns, linked with the Dystric Xeropsamments adsorption characteristics with long-term organic fertilization regime.
Methods
The mineral, chemical, and physical properties of the samples were characterized, including Langmuir adsorption and desorption kinetics. The P mobility was determined in one leaching experiment, in 20 cm soil columns. The topsoil (0–10 cm) layer was treated with organic (cattle, swine, goat, and hen manure) and inorganic fertilizers. Leaching was corresponding to 10 pore volumes (PV), 1 PV day−1. Aliquots of the leachate were collected to analyze P concentrations. After the leaching, the columns were sliced into 5-cm sections for the analysis of water-extractable P (WEP).
Results
The mineral source obtained higher leaching of P and between the organic sources the bovine and swine manure. The latter were the ones that had higher value of Pw in the soil after the leaching. Due to the increase of the adsorption capacity of P with depth, there was a reduction in the mobility of P, and an unbalance of Pw in the soil was found.
Conclusions
The mobility of P depends on the concentration of the soluble P soil or added material; moreover, the presence of Fe and Al oxides, even in small amounts, reduces the mobility of P in sandy soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-income small farming in Agrest is characterized by limited commercial use of nitrogen or phosphorus fertilizers. Soil fertility, therefore, solely relies on organic matter management (Nascimento et al. 2003), and that is the reason why organic fertilizers, especially manure, are widely used by farmers in that region. In the organic fertilizers being considered as a main source of nutrients to the plants, the soil conditioner effects have not been studied so far.
Furthermore, the use of manure in agriculture varies according to the availability, amount, and type of manure (cattle, swine, goat, and poultry). In the northeastern parts of Brazil, solid cattle manure and goat manure are frequently used in doses ranging from 4 to 20 Mg ha−1 (Sabourin et al. 2000; Silva and Menezes 2007; Galvão et al. 2008; Galvão and Salcedo 2009). In the south of the country, on the other hand, because the animal concentration of swine and cattle slurry is higher, doses around 80 m3 ha−1 are often used (Piovesan et al. 2009; Bertol et al. 2010; Ceretta et al. 2010).
Nutrients in animal manure are lower in concentration and unbalanced compared to commercial fertilizers, which hinder an even supply of nutrients to crops. The doses are usually based on demand for N of cultures, the size of the area or in the load capacity of the vehicle of transport common in the region and are generally multiples of 4 tons. Moreover, the wide variability of the chemical composition of manure may cause accumulation of one or more nutrients in the soil (Galvão et al. 2008), as the P, for example.
Accumulation of P, in the topsoil layers, has been reported in the literature (Ojekami et al. 2011; Kang et al. 2011; Rubæk et al. 2013). In Brazil, annual applications of manure in areas with Dystric Xeropsamments of sandy texture in sub-humid region in the northeastern Brazil (Agreste) have caused accumulation of nutrients in the surface layer (Silva and Menezes 2007; Galvão et al. 2008; Oliveira et al. 2011). Consequently, these nutrients move down the soil profile, including phosphorus (Galvão and Salcedo 2009; Xavier et al. 2009), which has little mobility in clayey soils. The accumulation of P in the topsoil increases the risk of its vertical displacement beyond the reach of the roots. This fact, in addition to representing an economic loss for the farmer, can become an environmental problem due to the contamination of subsurface water (Sharpley et al. 2004; Nelson et al. 2005; Galvão et al. 2008; Oliveira et al. 2011; Lee and Oa 2013).
To assess the risk, it is necessary to analyze the chemical composition of the soil, especially the content of poorly crystalline minerals represented by the oxalate-soluble Fe and Al fraction. Even sandy soils may contain small amounts of Fe and Al in their clay fraction, but that is enough to reduce the mobility of P (Sims et al. 1998). These elements are also components of oxides and hydroxides which are mainly responsible for the adsorption of P (Novais et al. 2007; Tarkalson and Leytem 2009; Fink et al. 2016).
For these reasons, the objective of this study was to evaluate phosphorus mobility in Dystric Xeropsamments fertilized with organic (cattle, swine, goat, and hen manure) and inorganic source materials for a long period by balancing phosphorus inputs and outputs and relating mobility to soil adsorption characteristics.
Methods
Soil collection and analysis
The soil samples were collected in municipality of Remigio, PB (6°59′14.9″ latitude S; 35°48′14.8″ longitude W) because of its fertilization regime. The chosen area has been fertilized exclusively with cattle manure for nearly 40 years. Part of the manure is produced locally, while the rest is purchased elsewhere in the area. However, little is known about the exact quantities used each year.
The climate of the region is characterized according to the scale of Koppen as As’, hot and humid, with a rainy season between February and August, with average rainfall of the last 20 years of 917.6 mm (AESA 2017). The soil was classified as Dystric Xeropsamments (Soil Survey Staff 2014). The samples were collected from an area of 2 × 3 m at two depths: 0–10 and 10–20 cm. Once collected, the samples were air dried, crushed, and sieved through 4-mm mesh for mounting the columns, and through a 2-mm mesh for mineralogical, chemical, and physical analysis.
The mineralogical analysis was performed using X-ray with Cu Kα radiation, 30 kV and 30 mA, with scan from 5° to 55° (2θ) on samples initially sieved through the 2-mm mesh, which after grinding were sieved through a 200 mesh (Table 1).
The following soil chemical attributes were determined: pH in water 1:2.5 (m/v), phosphorus extracted with Mehlich-1 (MEP) (EMBRAPA 2011), remaining P (P-rem) (Alvarez et al. 2000), and water-extractable phosphorus (WEP) which was extracted with deionized water and the supernatant filtered through a membrane of 0.45 µm, adapted by del Campillo et al. (1999). The concentrations of P in the extracts were determined according to Murphy and Riley (1962). The total organic carbon (TOC) was determined by wet oxidation–diffusion (Snyder and Trofymow 1984) and the water-extractable carbon (WEC) by the method proposed by Bartlett and Ross (1988) and adapted by Mendonça and Matos (2005). In addition to this, iron (Fe) and aluminum (Al) were extracted with ammonium oxalate and determined by atomic absorption (Schwertmann 1964) (Table 2). The following physical attributes were analyzed: particle size (densitometer method), particle density (Pd), bulk density (Bd), and total porosity (TP) (EMBRAPA 2011) (Table 3).
Phosphorus desorption kinetics was determined using paper strips impregnated with iron (Fe strips) in triplicate (van der Zee et al. 1987) for 1, 7, 22, 28, 43, 48, and 72 h. The results were processed by the SigmaPlot 10 program (Systat Software 2006) to estimate phosphorus surface adsorption (Qin) and the rate of desorption (Kd). The P-adsorption test was performed by the “batch” method with increasing concentrations of P (0, 5, 10, 20, 40, 60, and 100 mg L−1) with three replications and agitation for 24 h. This method was an adaptation of the method described by Farias et al. (2009). The amount of P in the solid phase was the difference between the P added to and recovered from the equilibrium solution. The P added to the solid phase (Qin) was found in the desorption experiment with Fe strips (van der Zee et al. 1987). Experimental data were adjusted using the adsorption equation proposed by Soares and Casagrande (2009):
where [P]ads is the concentration of adsorbed P (mg kg−1), Vsolution is the volume of solution (L), C0 is the initial concentration of P in the solution (mg L−1), Ce is the remaining concentration of P in the equilibrium solution (mg L−1), Msoil is the soil mass (kg), and Qin is the adsorbed phosphorus extracted with iron strips (mg kg−1).
The results of Pads and Ce were processed using the Langmuir equation with the aid of the SigmaPlot 10 program (Systat Software 2006).
P-leaching experiment with soil columns
To evaluate the vertical displacement of P for the organic or inorganic sources, polyvinyl chloride (PVC) columns were sequentially filled with soil layers. The experiment was conducted at the Departamento de Solos e Engenharia Rural do Centro de Ciências Agrárias—CCA. One assay was conducted with 20-cm columns containing soil from 0 to 10 and 10 to 20-cm layers.
The organic source materials were cattle, swine, goat, or hen manure, while the inorganic source material was a mixture of ammonium sulfate [(NH4)2SO4], monosodium phosphate (PO4H2Na), and potassium chloride (KCl).
After composting, the organic sources were dried, crushed, sieved through 2-mm mesh, and stored in airtight containers for chemical analysis. The analysis was determined: pH in water 1:10 (m/v); electric conductivity (EC) 1:10 (m/v); total P (Pt) obtained by digestion with H2SO4 and H2O2 (Thomas et al. 1967); P extracted with Mehlich-1 solution (MEP); P extracted with water (WEP) and the extracts determined by the method of Murphy and Riley (1962); WEC determined by the method of Mendonça and Matos (2005) adapted from Bartlett and Ross (1988); TOC obtained by wet oxidation–diffusion (Snyder and Trofymow 1984); and ash obtained by the method of EMBRAPA (2011) (Table 4).
The manure dose was calculated based on the content of Pt in the cattle manure, considering the application of 16 Mg ha−1 in the first 20 cm of soil, which is the mean annual dose applied in the region (Galvão and Salcedo 2009; Oliveira et al. 2011). However, we decided to apply 8 Mg ha−1 of cattle manure to the 0–10 cm layer to better observe P displacement. Based on this dose, an amount of P was set for other fertilizers, adjusting them to the same P input with cattle manure (35.3 kg P ha−1) (Table 5). The soil volume mixed with the sources was 1767 cm3, having a mass of 2650 g (0–10 cm).
The experimental design was completely randomized blocks with six treatments. Four organic source materials (cattle, swine, goat, and hen manure), one inorganic source, and a control were evaluated with three repetitions.
The columns were made of PVC pipes (150-mm diameter and 25-cm height), maintaining a 5-cm border from the top. First, the inner surface of each column was sanded. Second, the sand was washed with an acidic solution of 10% HCl. The sand was then glued with polychloroprene glue and synthetic resins to the inner wall of the tubes to increase soil adhesion to the wall and to avoid artificial preferential flow paths by the internal walls during the percolation of the solution. Finally, the bottoms of the columns were closed with a nylon screen of 77-μm mesh to prevent soil from escaping during the percolation of the solution and another nylon screen (50% shading) was used to maintain the soil in the column. Both were fastened with wire and PVC glue.
The organic sources were mixed with soil in the top layer (0–10 cm). The treated soil was moistened with distilled water until it reached 40% of the total pore volume (PV). Then, the setup was incubated for 15 days to stabilize the microbial activity, keeping moisture constant with daily weighings. After the incubation, the soil was air dried. This procedure was not carried out for the inorganic source.
Soil in the columns was prepared manually, assuming a mean bulk density of 1.5 g cm−3 found for this type of soil (Santos et al. 2012; Kang et al. 2011; Oliveira et al. 2011; Santos et al. 2010). The layer of 0–10 cm was gently set, because the soil when plowed is less dense. To dissipate the impact of the drops and evenly distribute the solution on the surface of the column, and to avoid the induction of preferential paths, three discs with a mesh of 50% shading capacity were placed on the soil with the leaning nozzle. After the assembly, six columns were placed on a wooden structure with funnels to collect the leachate into containers.
Rounds and volume of percolated solution
A hospital infusion set with tubing and flow regulators to supply CaCl2 at 0.001 mol L−1 was mounted on the columns (Roy and Dzomback 1996). The flow rate was 10 ± 1 cm3 min−1.
The volume of the applied solution was calculated using the pore volume (PV) of the columns (Eq. 2), which was 1500 cm3:
In total, ten pore volumes were applied to each column—1 PV per day. The displacement of the saline solution was vertical and downward. The collection of the column effluents started with the beginning of drainage, splitting each PV into three equal aliquots using calibrated containers. The aliquots were filtered through a 0.45-μm membrane. The concentration of P was determined according to Murphy and Riley (1962). Once the leaching stopped, the columns with the soil were dried in an oven at 50 °C and sliced evenly into 5-cm sections. The soil of each section was crushed, homogenized, and sieved through a 2-mm mesh. Finally, WEP values were obtained.
The balance of WEP in the columns was carried out by calculating its input and output. For this, the amounts of WEP already in the soil (soil WEPi) were added to the amounts in the source (source WEPi), and then subtracted from the leached amount, thus generating the final WEP (final. WEP). This value was compared with the final observed WEP (final obs. WEP) extracted with saline solution from the columns after leaching.
Statistical analysis
We performed analysis of variance (ANOVA) for total leachate and the comparison of means by Tukey test at 10% probability.
To evaluate the influence of the sources at each depth, we performed ANOVA and Tukey test (p < 0.1). In addition to ANOVA and Tukey (p < 0.1), a linear mixed model was created to compare the depths for each source. It estimates an additional error in analysis of variance to compensate for P variations between depths due to non-independence of the data, the lack of randomness, and autocorrelation of P content between depths. The program used for statistical analysis for SAS software 9.3 (2011) and for the construction of the figures the SigmaPlot 12.0.
Results and discussion
Leaching columns
The leaching of P was higher with the inorganic source starting at 9.67 mg of P for the first PV and decreasing to 0.26 mg for the tenth PV (Fig. 1a). However, P from the organic sources behaved differently, starting with a mean (n = 12) of 0.15 mg of leached P and increased to 0.27 mg at the tenth PV. Among the organic sources, P from swine manure leached the most, with increasing levels until the sixth PV, stabilization until the ninth PV, and increasing again after the tenth PV (Fig. 1b).
The leaching of P for the inorganic source had a logarithmic trend (r2 = 0.99) (Fig. 2a), while the organic sources and the control demonstrated a linear trend (r2 = 0.99) and did not differ (p < 0.1) (Fig. 2b).
Considering that the input of P was the same for all sources (35.3 kg ha−1 or 62.5 mg column−1 of P), the different behaviors of P from the organic and inorganic sources were due to the water-extractable P in the manures (Kang et al. 2011; Conti et al. 2015): 17.4, 16.0, 4.75, 6.18, and 62.5 mg of WEP column−1 added with cattle, swine, goat and hen manure, and the inorganic source, respectively.
Leached P for the inorganic source, which reached 22.3 mg or 34.4% of added P, was 18 times higher than the average of leached P for the organic materials (Fig. 2a). However, as the organic sources contained much less WEP, the percentage of leached P ranged between 6.6 and 18.8% of WEPi. On the other hand, the leached P showed relations with the WEP content in the sources, because the cattle and swine manure lost nearly double the value compared to the goat and hen manure (Fig. 2b). The physicochemical characteristics of the organic sources, such as lignin (Galvão et al. 2008) and the chemical forms of P in manure (soluble phosphate ion, nucleotides, phytin, Ca phosphates, and polyphosphates) certainly contributed to the variations of soluble P in the source materials.
Higher P losses for an inorganic source were also found by Kang et al. (2011). These authors also analyzed P leaching for organic and inorganic sources in columns with sandy soil. They observed higher P leachability for inorganic sources with 63 and 71% of P lost added, respectively, via triple superphosphate and potassium phosphate (KH2PO4) using approximately 23 PV. However, in this study, 10 PV were used and the losses reached 34.4% of P added via the inorganic source.
Perhaps, in our experiment, P from different sources was partially dragged down the profile with the percolating solution through artificial preferential flow paths within the soil column (Djodjic et al. 1999; Sharpley et al. 2003). We tried to prevent or reduce this effect by coating the inner surface of the columns with washed sand. Furthermore, there is consistency of data regarding the difference between the soluble inorganic and the organic source materials with lower volume of WEP. As already mentioned leached P for the organic sources shows correlation with WEPi. For these reasons, although the possibility of preferential paths cannot be totally ruled out, it was considered unlikely.
Another process in soils that may influence the leaching of P is the competition of P with organic matter compounds (organic acids and fulvic acid) for the same adsorption sites. As a result, P may remain in soil solution and be subject to leaching (Guppy et al. 2005a, b). In soils with low content of clay, organic and humic acids effectively block the adsorption sites, promoting lower P adsorption than in soils with high clay content (Andrade 2001). To reinforce the hypothesis of competition, non-tabulated results indicate that the soil placed in the columns contained, on average, 52.6 mg kg−1 of C per layer in water-soluble form. It is also possible that the percolation of organic P occurred (Galvão and Salcedo 2009) due to phosphatase-catalyzed hydrolysis during the collection of the percolated solution.
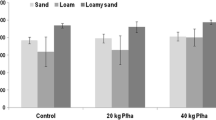
The concentrations of WEP remaining in soil columns after leaching varied depending on the P sources (Fig. 3). The lowest WEP concentrations were recorded for the control and for the soils in the columns treated with the inorganic source. The concentrations were uniform at different depths and lower in the case of the inorganic source, which is consistent with the high loss of P caused by leaching for this source (Figs. 1, 2). This is due to the mobility of this form of P in the column profile, which promoted lower accumulation of WEP in the soil resulting in concentrations similar to the control.
Distribution of concentrations of P extracted with water from 20-cm columns filled with Dystric Xeropsamments after application of 10 PV. Treatments followed by the same letter do not differ significantly by Tukey test (p < 0.1); lower case letters compare sources within each depth and upper case letters compare depths with each source. The sequence of the letters follows the order of the legend of the treatments (cattle, swine, goat, hen manure, inorganic source, and control)
However, leached P for the inorganic source reached 34% of the added P, indicating that part of the added P was not transformed into water-soluble forms. In fact, the WEP balance in the soil before and after leaching, including leached P, indicates 33 mg less of WEP in the soil column treated with the inorganic source (Table 6). The fact that 33 mg of P have gone indicates that there was an interaction of P with the soil matrix, even though the soil had a sandy texture with the predominance of quartz and feldspars in the layers of 0–20 cm (Table 1). However, it is noteworthy that there was a small contribution of hematite (Table 1) to decrease leaching and promote adsorption of P in columns (Sims et al. 1998; Tarkalson and Leytem 2009).
Among the organic sources, hen WEP showed the lowest concentrations in layers from 0 to 5 and 5 to 10 cm (Fig. 3), which is consistent with low WEP added via this source (Table 6). WEP for goat manure, however, which was similar to that of hen, did not differ (p < 0.1) from cattle and swine. Regarding the accumulated leached P (Fig. 2), the treatment with goat manure was between the treatment with hen manure, which was smaller, and swine and cattle manure, which were higher (Table 6). This trend of WEP for goat manure, consistent in leached P and the remaining WEP in the soil, suggests an increase in solubility of P from this source during the course of the experiment. The nature and mineralization of manure are very heterogeneous and are dependent on the species of the animal, its age, diet, and the physical and chemical conditions of the soil. These conditions dictate the speed of decomposition and consequent solubilization of organic and inorganic forms of nutrients (Tedesco et al. 1999). Souto et al. (2005), in evaluating manure decomposition in the semiarid region of Paraíba, found that the highest rates of decomposition were goat and cattle manure, which were strongly influenced by rainfall events during the trial period.
The comparison between layers demonstrates that WEP for the organic sources was similar (p < 0.10) in the first two layers due to the mixing of the sources to the soil was performed in the layer 0–10 cm, but at the depth of 10–15 cm, its concentration was between the concentration of the 5–10- and 15–20-cm layers (p < 0.10), showing a vertical displacement of WEP for the organic source. It is worth investigating the reason why WEP did not relocate with the same intensity as the WEP from the inorganic source. One possibility is that this soluble form of P was formed in situ during 25 days of the experiment (15 days of incubation and 10 days of leaching), as a result of mineralization of the organic sources. The balance between inputs and outputs of P, which indicate greater WEP inputs that the outputs, suggests this possibility (Table 6). Therefore, the hen manure seems to be more resistant to mineralization than the other three sources.
It is noteworthy that the total applied PV was 15 L. Comparing to the average rainfall over the last 20 years, which is 917 mm (AESA 2017), or equivalent to 17.6 L, the organic sources still accumulated P in the columns in a form which is available to plants, unlike the inorganic source.
Adsorption and desorption of P
Although clay content in the soil in this study was low (Table 3), its characteristics may favor P adsorption in its matrix (Tables 1, 2).
The adsorption and precipitation of P in soil are directly linked to the texture and soil pH (Lee and Oa 2013) and especially the levels of Fe and Al (Pinto 2012). The results of amorphous Fe and Al, extracted with ammonium oxalate, increased with depth (Table 3) and are positively correlated with clay content (r2 = 0.90 and 0.96, respectively). Kang et al. (2011) using a sandy soil (Feox = 0.20; Alox = 0.29; and clay = 21 mg g−1), similar to the one in our study, found adsorption of P in the soil, especially when observing the data from the treatment with the inorganic fertilizer. Tarkalson and Leytem (2009) also noted adsorption of P in a soil with clay content of 62 mg g−1. Therefore, the movement of P in soil profile may be reduced by small amounts of clay and/or Fe and Al oxides (Sims et al. 1998).
In the present study, the adsorption capacity of P adjusted for the Langmuir isotherm increased progressively with increasing depth (Fig. 4). Desorption values were consistent with the data adjusted by the Langmuir equation, because binding energy (KL) increased with the depth, and consequently reduced desorption kinetics of P (Kd) (Fig. 4 and Table 7). The relationship between Qmax and the amounts of Feox and Alox is more evident when the experimental data regarding Feox and Alox are used according to van der Zee and van Riemsdijk (1988). Based on studies by these authors, the ability of the poorly crystalline Fe and Al to adsorb P is approximately equal to half of the sum of their concentrations [(Feox and Alox)/2]. Table 7 shows the similarity between Qmax obtained by the Langmuir equation and according to van der Zee and van Riemsdijk (1988).
The binding energy and the degree of in saturation in 10–20 cm layers are higher than in the 0–10 cm layers, where P is believed to be transformed into forms which are not soluble in water (Table 8) (Gatiboni et al. 2008; Guardini et al. 2012; Conti et al. 2015).
The amounts of P desorbed by the Fe strips (Fe–P) showed an inverse relationship with Pads estimated by Langmuir equation for the soil before leaching (Table 8). Analyzing this apparent discrepancy, one should remember that Fe–P was experimentally determined, with the P desorbing directly from soil onto the Fe strips. Furthermore, Fe–P maintained correlation with the values of water soluble P in a 1:10 (m/v) ratio (Table 7, WEP initial and Qin). On the other hand, Pads estimated by Langmuir was calculated by adjusting the equation to the experimental data, and the parameters of this equation, especially KL, showed considerable uncertainty (Table 7). Furthermore, Pads estimated by Langmuir was derived from the difference between P added to an aqueous suspension and P found in the solution after 24 h of stirring. Thus, by inference, there is no reversibility in this phenomenon. It was found that, as the adsorption capacity of the solid phase increased, the resistance of Pads to return to the solution also increased (Tiessen et al. 1993). Therefore, although the amounts of Pads by Langmuir estimated the added P with reasonable approximation, it is not possible to draw conclusions as to the availability of this form of P for the plants.
The results of leaching and distribution of P in the columns show that there was a translocation of P from layers with lower binding energy to layers with higher binding energy, with the soil acting as a sink. Studies have shown that long-term fertilization with manure can promote changes in P adsorption influencing its dislocation into deeper layers of the soil profile (del Campillo et al. 1999; Silva and Menezes 2007; Galvão et al. 2008; Xavier et al. 2009; Tarkalson and Leytem 2009; Ojekami et al. 2011; Abdala et al. 2012; Bhattacharyya et al. 2015). In this regard, leaching of P may occur in soils saturated with P, even if there is no excessive input of P. Nelson et al. (2005) found higher values of leached P than P in the inputs for soils saturated with P when precipitation was above the average, indicating that desorption of P contributed to 50% of leached P.
The saturation of the adsorption sites in soil cannot be totally formed by phosphate ions. Although the phosphate ions are adsorbed preferentially, they have to compete with organic acids (Andrade 2001; Guppy et al. 2005a, b; Dotaniya et al. 2016). In this case, P leaching may occur even if the soil is not completely saturated with P.
According to del Campillo et al. (1999), 13–26 is an acceptable range for good crop development. The remaining concentrations of WEP in the 20-cm soil column for the cattle and swine manure were within this range, even after the application of the percolating solution equivalent to 851 mm of rainfall. This shows that these two source materials, the cattle and swine manure, provide high amounts of readily available nutrients for plants, because P leached the most from these sources, among all organic sources, and showed the highest amount of WEP remaining in the 20-cm soil columns.
Conclusions
The leaching of P in soil is dependent on the concentration of soluble P in the soil, whatever its source material from soil or added via organic or inorganic source. In this study occurred the leaching of P in columns of 20 cm, in which the greatest amount of P was leached from the inorganic source when compared with the organic sources with lower expression.
The availability of P by means of organic sources, manure from cattle, and pigs were the most made P, having been observed the greatest quantities in leachate (2.46 and 2.68 mg P) and recovered in the soil (48.2- and 49.8-mg WEP) after leaching, for the columns of 20 cm.
The reduction in leaching of P in the columns with 20 cm was related to the increase of the adsorption capacity of P from the soil in layers of 0–10 cm (93 mg kg−1) in relation with the layers 10–20 cm (128 mg kg−1). This indicated a significant reduction of the displacement of P. This suggests that the Fe and Al oxides present in a sandy soil, even in small quantities, decrease the mobility of P in the soil.
The model proposed by van der Zee and van Riemsdijk (1988) seems to be adequate to estimate the maximum P-adsorption capacity also in soil with sandy texture to the southern hemisphere; however, this confirmation needs further studies. Although the results of this study reveal adsorption of P in the layer of 10–20 cm in a Dystric Xeropsamments, new investigations about the saturation of P in these soils and about the influence of organic matter in the displacement of P in soil are still needed.
References
Abdala DB, Ghosh AK, Silva IR, Novais RF, Venegas VHA (2012) Phosphorus saturation of a tropical soil and related P leaching caused by poultry litter addition. Agric Ecosyst Environ 162:15–23. https://doi.org/10.1016/j.agee.2012.08.004
Agência Executiva de gestão das águas do estado da Paraíba—AESA (2017) Monitoramento pluviométrico. http://site2.aesa.pb.gov.br/aesa/monitoramentoPluviometria.do?metodo=listarAnosChuvasAnuais. Accessed 25 Oct 2017
Alvarez VVH, Novais RF, Dias LE, Oliveira JA (2000) Determinação e uso do fósforo remanescente. Boletim informativo da SBCS 25:27–32
Andrade FV (2001) Ácidos orgânicos e adsorção de fosfato em Latossolos. Tese, Universidade Federal de Viçosa
Bartlett RJ, Ross DN (1988) Colorimetric determination of oxidizable carbon in acid soil solutions. Soil Sci Soc Am J 52:1191–1192. https://doi.org/10.2136/sssaj1988.03615995005200040055x
Bertol OJ, Fey E, Ravaretto N, Lavoranti OJ, Rizzi NE (2010) Mobilidade de P, Cu e Zn em colunas de solo sob sistema de semeadura direta submetido às adubações mineral e orgânica. Rev Bras Cienc Solo 34:1841–1850. https://doi.org/10.1590/S0100-06832010000600008
Bhattacharyya P, Nayak AK, Shahid M, Tripathi R, Mohanty S, Kumar A, Raja R, Panda BB, Lal B, Gautam P, Swain CK, Rou KS, Dash PK (2015) Effects of 42-year long-term fertilizer management on soil phosphorus availability, fractionation, adsorption–desorption isotherm and plant uptake in flooded tropical rice. Crop J 3:387–395. https://doi.org/10.1016/j.cj.2015.03.009
Ceretta CA, Lorensini F, Brunetto G, Girotto E, Gatiboni LC, Lourenzi CR, Tiecher TL, Deconti L, Trentin G, Miotto A (2010) Frações de fósforo no solo após sucessivas aplicações de dejetos de suíno em plantio direto. Pesq Agropec Bras 45:593–602. https://doi.org/10.1590/S0100-204X2010000600009
Conti L, Ceretta CA, Ferreira PAA, Lorensini F, Lourenzi CR, Vidal RF, Tassinari A, Brunetto G (2015) Effects of pig slurry application and crops on phosphorus content in soil and the chemical species in solution. R Bras Ci Solo 39:774–787. https://doi.org/10.1590/01000683rbcs20140452
del Campillo MC, van der Zee SEATM, Torrent J (1999) Modelling long-term phosphorus leaching and changes in phosphorus fertility in excessively fertilized acid sandy soils. Eur J Soil Sci 50:391–399. https://doi.org/10.1046/j.1365-2389.1999.00244.x
Djodjic F, Bergström L, Ulén B, Shirmohammadi A (1999) Mode of transport of surface-applied phosphorus-33 through a clay and sandy soil. J Environ Qual 28:1273–1282. https://doi.org/10.2134/jeq1999.00472425002800040031x
Dotaniya ML, Datta SC, Biswas DR, Dotaniya CK, Meena BL, Rajendiran S, Regar KL, Lata M (2016) Use of sugarcane industrial by-products for improving sugarcane productivity and soil health. Int J Recycl Org Waste Agric 5:185–194. https://doi.org/10.1007/s40093-016-0132-8
Embrapa-Empresa Brasileira de Pesquisa Agropecuária (2011) Manual de Métodos de Análise de Solo. Embrapa Solos EMBRAPA—CNPS, Rio de Janeiro
Farias DR, Oliveira FHT, Santos D, Arruda JA, Hoffmann RB, Novais RF (2009) Fósforo em solos representativos do estado da Paraíba. I—Isotermas de adsorção e medidas do fator capacidade de fósforo. Rev Bras Cienc Solo 33:623–632. https://doi.org/10.1590/S0100-06832009000300015
Fink JR, Inda AV, Bavaresco J, Barrón V, Torrent J, Bayer C (2016) Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Till Res 155:62–68. https://doi.org/10.1016/j.still.2015.07.017
Galvão SRS, Salcedo IH (2009) Soil phosphorus fractions in sandy soils amended with cattle manure for long periods. Rev Bras Cienc Solo 33:613–622. https://doi.org/10.1590/S0100-06832009000300014
Galvão SRS, Salcedo IH, Oliveira FF (2008) Acumulação de nutrientes em solos arenosos adubados com esterco bovino. Pesq Agropec Bras 43:99–105. https://doi.org/10.1590/S0100-204X2008000100013
Gatiboni LC, Brunetto G, Kaminski J, Rheinheimer DS, Ceretta CA, Basso CJ (2008) Formas de fósforo no solo após sucessivas adições de dejeto líquido de suínos em pastagem natural. R Bras Ci Solo 32:1753–1761. https://doi.org/10.1590/s0100-06832008000400040
Guardini R, Comin JJ, Rheinheimer DS, Gatiboni LC, Tiecher T, Schmitt DE, Bender MA, Belli Filho P, Oliveira PAV, Brunetto G (2012) Phosphorus accumulation and pollution potential in a Hapludult fertilized with pig manure R Bras Ci Solo 36:1333–1342. https://doi.org/10.1590/s0100-06832012000400027
Guppy CN, Menzies NW, Blamey FPC, Moody PW (2005a) Do decomposing organic matter residues reduce phosphorus sorption in highly weathered soil? Soil Sci Soc Am J 69:1405–1411. https://doi.org/10.2136/sssaj2004.0266
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005b) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Aust J Soil Res 43:189–202. https://doi.org/10.1071/SR04049
Kang J, Amoozegar A, Hesterberg D, Osmond DL (2011) Phosphorus leaching in a sandy soil as affected by organic and inorganic fertilizer sources. Geoderma 161:194–201. https://doi.org/10.1016/j.geoderma.2010.12.019
Lee Y, Oa SW (2013) Nutrient transport characteristics of livestock manure in a farmland. Int J Recycl Org Waste Agric 2:1. https://doi.org/10.1186/2251-7715-2-1
Mendonça ES, Matos ES (2005) Matéria orgânica do solo: Métodos de análise. Universidade Federal de Viçosa, Viçosa
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural water. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nascimento JT, Silva IF, Santiago RD, Silva Neto LF (2003) Efeito de leguminosas nas características químicas e matéria orgânica de um solo degradado. Rev Bras Eng Agr Amb 7:457–462. https://doi.org/10.1590/S1415-43662003000300008
Nelson NO, Parson JE, Mikkelsen RL (2005) Field-scale evaluation of phosphorus leaching in acid sandy soils receiving swine waste. J Environ Qual 34:2024–2035. https://doi.org/10.2134/jeq2004.0445
Novais RF, Smyth TJ, Nunes FN (2007) Fósforo. In: Novais RF, Alvarez VVH, Barros NF, Fontes RL, Cantaruti RB, Neves JCL (eds) Fertilidade do solo. SBCS, Viçosa, pp 471–550
Ojekami A, Ige D, Hao X, Akinremi O (2011) Phosphorus mobility in a soil with long term manure application. J Agric Sci 3:25–38. https://doi.org/10.5539/jas.v3n3p25
Oliveira FF, Salcedo IH, Galvão SR (2011) Adubação orgânica e inorgânica de batatinha em solos arenosos: produtividade, nutrientes na planta e lixiviação. Rev Bras Eng Agr Amb 15:1228–1234. https://doi.org/10.1590/S1415-43662011001200003
Pinto F A (2012) Sorção e dessorção de fósforo em solos de cerrado. Dissertation, Universidade Federal de Goiás
Piovesan RP, Favaretto N, Pauletti V, Motta ACV, Reissmann CB (2009) Perdas de nutrientes via subsuperfície em colunas de solo sob fertilização mineral e orgânica. Rev Bras Cienc Solo 33:757–766. https://doi.org/10.1590/S0100-06832009000400002
Roy B, Dzomback DA (1996) Colloid release and transport processes in natural and model porous media. Colloid Surface A 107:245–262. https://doi.org/10.1016/0927-7757(95)03367-X
Rubæk GH, Kristensen K, Olesen SE, Østergaard HS (2013) Phosphorus accumulation and spatial distribution in agricultural soils in Denmark. Geoderma 209–210:241–250. https://doi.org/10.1016/j.geoderma.2013.06.022
Sabourin E, Silveira LM, Tonneau JP, Sidersky P (2000) Fertilidade e agricultura familiar no Agreste Paraibano: Um estudo sobre o manejo da biomassa. CIRAD-TERRA/ASPTA, Esperança
Santos JS, Lima VLA, Júnior JCFB, Silva LVBD, Azevedo CAV (2010) Mobilidade de solutos em colunas de solo com água residuária doméstica de suinocultura. Rev Bras Eng Agr Amb 14:1226–1233. https://doi.org/10.1590/S1415-43662010001100013
Santos JCB, Souza Junior VSS, Corrêa MM, Ribeiro MR, Almeida MC, Borges LEP (2012) Caracterização de Neossolos Regolíticos da região semiárida do Estado de Pernambuco. Rev Bras Cienc Solo 36:683–695. https://doi.org/10.1590/S0100-06832012000300001
SAS. SAS/STAT 9.3 (2011) User’s guide. SAS Institute, Cary
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch Extraktion mit saurer Ammoniumoxalat-Lösung. Z Pflanzenernaehr Bodenkd 105:194–202
Sharpley AN, Weld JL, Beegle DB, Kleinman PJA, Gburek WJ, Moore PA Jr, Mullins G (2003) Development of phosphorus indices for nutrient management planning strategies in the United States. J Soil Water Conserv 58:137–152
Sharpley AN, McDowell RW, Kleinnman JA (2004) Amounts, forms, and solubility of phosphorus in soils receiving manure. Soil Sci Soc Am J 68:2048–2057. https://doi.org/10.2136/sssaj2004.2048
Silva TO, Menezes RSC (2007) Adubação orgânica da batata com esterco e, ou, Crotalaria juncea. II—Disponibilidade de N, P e K no solo ao longo do ciclo de cultivo. Rev Bras Cienc Solo 31:51–61. https://doi.org/10.1590/S0100-06832007000100006
Sims JT, Simard RR, Joern BC (1998) Phosphorus loss in agricultural drainage: historical perspective and current research. J Environ Qual 27:227–293. https://doi.org/10.2134/jeq1998.00472425002700020006x
Snyder JD, Trofymow JA (1984) A rapid accurate wet oxidation diffusion procedure for determining organic and inorganic carbon in plant and soil sample. Commun Soil Sci Plant Anal 15:587–597. https://doi.org/10.1080/00103628409367499
Soares MR, Casagrande JC (2009) Adsorção e Modelos. In: Ribeiro MR et al (eds) Tópicos em Ciência do Solo. SBCS, Viçosa, pp 71–201
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. USDA-Natural Resources Conservation Service, Washington, DC
Souto PC, Souto JS, Santos RV, Araújo GT, Souto LS (2005) Decomposição de estercos dispostos em diferentes profundidades em área degradada no semi-árido da Paraíba. Rev Bras Cienc Solo 29:125–130. https://doi.org/10.1590/S0100-06832005000100014
Systat Software (2006) SigmaPlot for windows. Version 10.0. Systat Software, San Jose
Tarkalson DD, Leytem AB (2009) Phosphorus mobility in soil columns treated with dairy manures and commercial fertilizer. Soil Sci 174:73–80. https://doi.org/10.1097/SS.0b013e31819750la
Tedesco MJ, Selbach PA, Gianello C, Camargo FA (1999) Resíduos orgânicos no solo e os impactos no ambiente. In: Santos GA, Camargo FA (eds) Fundamentos da matéria orgânica do solo: ecossistemas tropicais e subtropicais. Gênesis, Porto Alegre, pp 159–192
Thomas RL, Sheard RW, Moyer JR (1967) Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron J 59:240–243. https://doi.org/10.2134/agronj1967.00021962005900030010x
Tiessen H, Abekoe MK, Salcedo IH, Owusu-Bennoah E (1993) Reversibility of phosphorus sorption by ferruginous nodules. Plant Soil 153:113–124
van der Zee SEATM, van Riemsdijk WH (1988) Model for long-term phosphate reaction kinetics in soil. J Environ Qual 17:35–41. https://doi.org/10.2134/jeq1988.00472425001700010005x
van der Zee SEATM, Fokkink LGJ, van Riemsdijk WH (1987) A new technique for assessment of reversibly adsorbed phosphate. Soil Sci Soc Am J 51:599–604. https://doi.org/10.2136/sssaj1987.03615995005100030009x
Xavier FAS, Oliveira TS, Andrade FV, Mendonça ES (2009) Phosphorus fractionation in a sandy soil under organic agriculture in Northeastern Brazil. Geoderma 151:417–423. https://doi.org/10.1016/j.geoderma.2009.05.007
Acknowledgements
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), by granting the Master’s scholarship to the first author. The Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through research funding and to Dr. Bruno O. Dias for the support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to urisdictional claims in published maps and institutional affiliations.
In memoriam of Ignacio Hernán Salcedo.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Azevedo, R.P., Salcedo, I.H., Lima, P.A. et al. Mobility of phosphorus from organic and inorganic source materials in a sandy soil. Int J Recycl Org Waste Agricult 7, 153–163 (2018). https://doi.org/10.1007/s40093-018-0201-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-018-0201-2