Abstract
Purpose
The implications of increased applications of high quality cattle manure to agricultural systems in Africa on N2O emissions are still only partially understood.
Methods
A field experiment was carried out in a wetland in central Zimbabwe to determine the effects of cattle manure quality on emissions of N2O during the growing seasons of rape and tomato crops. The static chamber and gas chromatography techniques were used to capture and measure fluxes of N2O.
Results
The substitution of low N by high N manure significantly increased N2O fluxes and total N lost through N2O emission. Emissions of N2O increase with increasing content of N in applied manure.
Conclusions
Given that N2O in agricultural soil is produced predominantly through the microbial transformations of inorganic N, the potential of a soil to emit N2O increases with the increasing availability of N and consequently the N content in applied manure. The applications of lower rate of high and low N manures were followed by lower emissions of N2O, a result that is favorable to the objective of lowering the contribution of agricultural sources to the global greenhouse gas emissions. The loss of N in emissions of N2O expressed per unit mass of harvested dry matter yield decreases with increasing manure application, dry matter yield and N uptake. Improved agronomic practices for increased crop productivity can be used as a mitigation factor for reducing the contribution of agriculture in the global emissions of N2O.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In developing countries, the increasing prices of inorganic fertilizers coupled with growing concerns for sustaining soil productivity have led to renewed interest in the use of organic manures as fertility-restorer inputs (Mapfumo et al. 2007; Ouedraogo et al. 2007; Masvaya et al. 2011). Manures are a vital resource not only for supplying plant nutrients but also for replenishing organic matter content of agricultural soils particularly in the tropics (Groot et al. 2006; Materechera 2010).
In Zimbabwe, cattle manures are largely prepared by subjecting cattle excreta together with maize stover to prolonged storage. This increases C stabilization resulting in a low rate of N mineralization (Zingore et al. 2008; Materechera 2010; Wuta and Nyamugafata 2012). The quality of cattle manure reflects the average concentrations of nutrients in the livestock diet. Livestock diet from communal grazing areas in Zimbabwe are, in most cases, of poor forage value (Nyamangara and Nyagumbo 2010). Cattle manures from smallholder areas in Zimbabwe are generally regarded to be of lower quality than manures from commercial farming areas (Whitlow 1985; Zingore et al. 2008).
The quality of organic material applied to a soil determines the rate of release of mineral nitrogen (N), which may be subjected to loss by nitrous oxide (N2O) emission during denitrification under anaerobic soil conditions (Dobbie et al. 1999; Markewich et al. 2010; Smith 2012). Although there is a general trend relating net mineralization/immobilization to the carbon to nitrogen (C:N) ratio, there is no critical precise value, which marks the reversal from immobilization to mineralization (Mafongoya et al. 1998; Yates et al. 2006; Van der Meer 2008). The lignin and polyphenol content of organic manure have a major impact on rate and direction of decomposition (Mtambanengwe et al. 1998; Silva et al. 2005).
Addition of cattle manures to soils in sub-tropical Africa increases the amount of readily decomposable organic matter. This enhances the potential for denitrification and increased emissions of N2O gas through a general stimulation of microbial respiration, causing rapid oxygen consumption and consequently an increase of anaerobic conditions (Johnson et al. 2005; Jassal et al. 2011). N2O is a greenhouse and ozone-depleting gas (Mosier and Kroetze 1999; Vasileiadou et al. 2011) whose atmospheric concentration is currently increasing at a rate of approximately 0.4 % per annum (Intergovernmental Panel for Climate Change (IPCC) 2001). Although very much a trace component of the Earth’s atmosphere, it is estimated to account for some 6 % of the greenhouse warming (Snyder et al. 2009; Smith 2012). Formation in the soil as a side-product of the biological processes of nitrification and denitrification is believed to account for as much as 90 % of the global atmospheric N2O (Ma et al. 2007; Snyder et al. 2009). Direct emissions of N2O from agricultural soils have increased substantially over the last few decades (Ma et al. 2007), in parallel with increasing use of organic manures (Conrad et al. 1983; Johnson et al. 2005; Saggar 2010; Lin et al. 2011). These factors are exacerbated by there being no chemical sinks for N2O in the troposphere, resulting in the mean residence time in the atmosphere of about 150 years (Munoz et al. 2010; Saggar 2010). On average, 0.3–2.25 % of applied N to agricultural soils may be emitted as N2O (Smith et al. 1997; Mosier et al. 2003; Saggar 2010).
Existing data on emissions of N2O are extracted from research generated in western Europe, north America and south-east Asia (Kroetze et al. 2003) despite the fact that the tropics and subtropics contribute greatly to the emissions (Grenon et al. 2004; Billy et al. 2010), particularly since 51 % of world soils are in these climate zones (Mosier et al. 2003). There are few published studies from Africa examining the response function relating N2O emissions to cattle manure quality (Hickman et al. 2011). Current estimates of N2O emissions from African agriculture at the national, regional, and continental scale are mostly based on the IPCC Guidelines, which implicitly ignore important characteristics of African soils and management practices of smallholder farmers, both of which could alter emissions substantially (Smith 2012). In compiling the global greenhouse gas (GHG) inventories, it is necessary to use country-specific data, where available, for the activity data and N2O emission factors (Van Der Salm et al. 2006). There is considerable scope for the present IPCC default emission factor for N2O of 1.2–2 % of the N applied default emission factor to change as more data become available from sub-tropical African regions (Snyder et al. 2009).
Consequently, a two-season study was carried out at a wetland site in central Zimbabwe to determine the effect of cattle manure application rate and quality (content of N) on mineral N concentrations in soil (ammonium N and nitrate N) (NH4–N + NO3–N), dry matter yield, N uptake by rape and tomato and N2O emissions from wetland soil. In this study, it was hypothesized that mineral N concentrations in soil, dry matter yield of rape and tomato, N uptake and emissions of N2O from wetland soil amended with cattle manure increases with increasing content of N in applied manure (manure quality) and rate of application.
Materials and methods
Site description
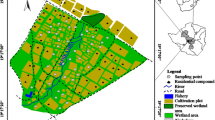
The study was conducted between 2007 and 2009 in a typical wetland garden at Dufuya (19°17′S; 29°21′E, 1260 m above sea level) wetlands in Chief Sogwala area of Lower Gweru Communal Lands, about 42 km west of the city of Gweru in Zimbabwe (Fig. 1). The field experimental site is in Agro-ecological Region III, which receives total rainfall ranging from 650 to 800 mm per annum (average 725 mm) and mean annual temperature is 21 °C with insignificant frost occurrence in the months of June and July (Vincent and Thomas 1960; Mugandani et al. 2012; Ministry of Environment and Natural Resources Management 2010). Rainfall occurs during a single rainy season extending from November to April.
The experimental soil is a deeply weathered course textured loamy sand topsoil over sandy loam subsoil derived from granite and classified as Udic Kandiustalf (United States Department of Agriculture, USDA) and Gleyic Luvisol (Food and Agriculture Organization (FAO)) (FAO 1988; Nyamapfene 1991; Soil Survey Staff 1992). The soil is perennially moist in part of the profile and smallholder farmers have established vegetable gardens along the wetland.
Characterization of experimental soil
Initial soil characterization was done by collecting twenty soil samples from randomly selected points of the experimental site at depths of 0–20; 20–60 and 60–100 cm using a soil auger. The soil samples were mixed thoroughly in a clean plastic bucket to obtain a composite sample. The composite sample was air-dried, sieved (<2 mm) and characterized (Table 1). Organic carbon (C) in soil was determined using the Walkely and Black method (Nelson and Sommers 1986). Soil texture was determined by the Bouyocous hydrometer method (Bouyoucos 1965). Soil bulk density was determined by the core method (Black and Hartge 1986). The soil cores were oven-dried at 105 °C (to constant weight) for determination of mean gravimetric water content. Taking particle density (Pd) of soil to be 2.65 g cm−3 total porosity was calculated and recorded. Total N in soil was measured by the Kjeldahl method described by Bremner (1996) (Table 1).
Experimental manure
The smallholder farmers at Dufuya wetlands practice intensive tomato and rape production in small gardens under small scale irrigation (Owen et al. 1995). Because of lack of availability and higher cost of chemical fertilizers, smallholder farmers have resorted to use of cattle manure which are readily available. The majority of the wetland smallholder farmers at Dufuya have dual land holdings. They own small holdings in the Dufuya community as well as plots in the adjacent Vungu commercial farming area (Fig. 1). The wetland vegetable farmers apply cattle manure collected from the two areas. The quality of cattle manure (content of N) collected from the adjacent areas differs due to differences in the grazing quality (Zingore et al. 2008; Nyamangara and Nyagumbo 2010; Wuta and Nyamugafata 2012). The two types of cattle manure were used in the field plot namely: cattle manure collected from a homestead in the surrounding communal area (smallholder cattle manure, high N (HN) manure) and manure collected from a farm in the adjacent Vungu commercial farming area (commercial farming area manure, low N (LN) manure). High rates of cattle manure applications are used to avoid yield depression due to nutrient deficiency (Owen et al. 1995; De Lannoy 2001). Usually, 15 Mg/ha of cattle manure is applied by wetland farmers with limited number of cattle (less than 6). On average, 30 Mg cattle manure/ha is applied by wetland farmers with larger cattle herds (more than 6). The manure application rates were used as treatments in the experiments to capture the common farmer practice. The manure used in the experiments was stored in the shade in cattle holding pens for a period 32 weeks before application.
Samples from the two types of cattle manure were analyzed for organic C (Nelson and Sommers 1982), total N using the Kjeldahl procedure (Stevenson 1982; Bremner and Mulvaney 1982), soil, and ash content.
Experimental design and treatments
Two experiments were used to determine the effect of cattle manure quality and application rates on N2O emission with three of the following treatments for each experiment: Experiment 1: (1) Control (unamended); (2) 15 Mg high N manure/ha; (3) 30 Mg high manure N/ha. Experiment 2: (1) Control (unamended); (2) 15 Mg low N manure/ha; (3) 30 Mg low N manure/ha. A completely randomized block design with four replications was used in which the slope was the blocking factor for each experiment. Cattle manure was applied only once in the study period before planting of the first tomato crop. The manure was evenly broadcast on the respective plots and then incorporated into the topsoil 7 days before transplanting the first crop. Comparison of the corresponding measured variables in plots subjected to high and low N manure applications was established by performing t test for unpaired samples using the GenStat package. Measured corresponding variables in the control plots for both experiments were not significantly different (p > 0.05) and therefore only one control measured variable was shown in the figures and tables data display methods.
Land preparation and crop management
The land was prepared by digging using hand hoes to a depth of 30 cm and then leveling using a rake. Plots raised to a height of 15 cm, which measured 5 by 1.5 m, were then carefully marked out. The distance between the plots was 60 cm. Small 20 cm high ridges were established around each plot to avoid cross-contamination by surface run-off. Tomato and rape crops were used as test crops in the study. The cropping sequence in the field experiment was: September–December 2007 first tomato, January–March 2008 first rape, April–July 2008s tomato and September–November 2008 s rape crops. Spacing between rows was 30 and 15 cm within the rows for the rape crop. For the tomato crop the plant spacing was 90 cm between rows and 80 cm within rows.
Weather conditions
Rainfall data were collected daily at 10.00 h from a rain gauge at the study site. Maximum and minimum daily temperatures at the study site were gap-filled using the department of Agricultural Technical and Extension Services (AGRITEX) meteorological data at Sogwala (19°17′S; 29°21′E) rural service centre located 2 km west of the study site. The meteorological station records daily weather data.
Static chamber setup and N2O flux measurement
Nitrous oxide emissions from soil were trapped using open-bottomed polythene static chambers with a trapping area of 0.03 m2 (18 cm internal diameter, 20 cm height, 1.5 mm wall thickness) described by Holland et al. (1999) and Meyer et al. (2001). There were seven gas sampling campaigns at 14-day interval for the tomato crop. Six gas sampling events were performed at 14-day interval for the rape crop. The gas sampling was replicated four times for each treatment. Gas sampling was done at time 0 min to obtain the start values of atmospheric concentration of N2O in the static chamber head space and after 30 and 60 min (Mathias et al. 1980; Kaiser et al. 1996). The gas samples were analyzed for N2O concentration by means of a Varian Model 3400 gas chromatograph (Walnut Creek, CA, USA) as described by Mosier and Mack (1980) and Galle et al. (2003). Nitrous oxide fluxes (F n) were calculated using the Hutchinson and Livingston (1993) model:
where \(\delta C_{\text{n}} /\delta t\) is the rate of change in N2O concentration (µmol/mol/min), V is the chamber headspace volume (m3), Mn is the molecular weight of N2O (44 g/mol), A is the surface area (m2) and V mol is the volume of one mole of gas at 20 °C (0.024 m3/mol). Further conversions were performed to calculate F n fluxes in g/ha/day as follows (Eq. 2):
Total N lost as N2O (N kg/ha) was calculated using Eq. 3:
where T is the number of days with similar daily N2O emissions rates (cumulative emissions calculated by the mean values between two sampling events multiplied by the time interval in days between them) and 28/44 is the conversion ratio for converting N2O molar mass to N content.
Soil mineral N measurements
At the same time that gas samples were collected, soil samples (n = 4) also collected from the plots and analyzed for NH4–N and NO3–N. Both analyses were performed using an Alpkem 3550 Flow Injector Analyzer (01 Analytical, College Station, TX, USA) using colorimetric techniques (Robertson et al. 1999).
Dry matter yield and N uptake
Four randomly selected plants were chosen and labeled in each plot for crop biomass sampling. All rape leaves and tomato fruits that reached horticultural maturity were harvested from the selected plants at every harvesting event and taken to the laboratory. The samples were rinsed; oven dried at 65 °C for 24 h and kept in a dry place. At the end of the growing season, the aboveground biomass of the selected plants was summed up. The composite samples were then ground to pass a 2 mm sieve and analyzed for N concentration semi-micro Kjeldahl procedure (Bremner and Mulvaney 1982). Total uptake of N was determined by multiplying the N concentration with dry matter yield as follows (Eq. 4):
where [N] is content of N in mg/g dry matter and DM is dry matter yield in Mg/ha.
Statistical analysis
Treatment effects on measured variables in each experiment were analyzed using one-way ANOVA (GenStat Discovery Edition 3 2003). Differences between treatment means were judged significant at p ≤ 0.05 as determined by Fisher’s protected least significant difference test. Flux data were log-transformed if needed, to normalize the distributions before the statistical analysis. Mean separation was performed using the LSD since there were not more than three treatments in each set of experiment. Statistical significance of the differences between measured variables in plots subjected to high N and low N manure applications was established by performing t test for unpaired samples using the GenStat package. The Pearson coefficients of determination between measured variables and their r 2 values were computed using Microsoft Excel. Significance of correlations between selected variables was established using a linear model GenStat analysis of correlation at 5 % level.
Results and discussion
Weather conditions
The 2007–2008 rain season started at the end of September. About 98 % (792 mm) of the total rainfall (808.2 mm) was received in the first half of the season (September–January; Fig. 2). The first tomato and first rape crops were cultivated during the first half of the 2007–2008.
Cattle manure quality
Table 2 shows the selected chemical characteristics of high N (HN) and low N (LN) cattle manure used in the study. The term ‘quality of cattle manure’ is wider than just the content of N and C:N ratio. In this study, cattle manure quality is confined to content of N and C:N ratio. Carbon and nitrogen contents in the high N cattle manure were 2.5 and 2.7 times more than that in low N manure, respectively. The C to N ratios of the high and low N manure were 17: 1 and 18: 1, respectively.
A comparatively large body of knowledge has been generated in Zimbabwe which points to the fact that smallholder cattle manures are generally of poor quality due to inadequate and low quality grazing and inappropriate handling of the manure in kraals (Zingore et al. 2008; Wuta and Nyamugafata 2012). Results of the chemical analysis of the two types of cattle manures showed quite the opposite of these previous findings (Table 2). The smallholder cattle manure collected from a homestead within the wetland community contained more N than that collected from an adjacent commercial farming area. This was due in greater part to the perennially green grazing of high forage value around the wetland area, which acted as an effective supplement for the poor grazing in the dry land portions of the Dufuya community. This implied that high N cattle manure collected within the Dufuya community essentially posed a greater potential to supply mineralized N and the associated capacity to enhance emissions of N2O to the atmosphere where it has detrimental effects on the processes of global warming and ozone depletion (Vasileiadou et al. 2011).
Mineral N concentration in soil
Results shown in Figs. 3 and 4 indicate that cattle manure quality (manure N content) and the rates of applications had a significant effect (p < 0.05) on the concentration of mineral N in soil during the growing seasons of the four vegetable crops. However, treatment differences in the concentrations of mineral N in soil became apparent in this study 42 days after planting of the first tomato crop (Figs. 3a, 4a). Mineral N concentrations in soil from plots subjected to application of 15 and 30 Mg/ha of high N manure were 146–171 and 143–308 % in excess of those recorded in the control plots. The concentration of mineral N in soil amended with 15 and 30 Mg/ha of low N manure exceeded those recorded in control plots by 53–61 and 84–157 %, respectively. Increasing the rates of application from 15 to 30 Mg/ha of high and low N manure significantly (p < 0.05) increased the concentration of mineral N in soil by 50–62 and 59–67 %, respectively.
Except for the first rape crop (Figs. 3b, 4b) the application of 15 and 30 Mg of high and low N manure/ha considerably increased the content of NH4–N and NO3–N in soil at the beginning of the growing season of the vegetable crops before gradually decreasing at the end of the season. Soil NO3–N concentrations during the September 2007 to November 2008 period followed a pattern that comparatively matched the rainfall events at Dufuya (Figs. 2, 4). Higher NO3–N concentrations were recorded during the drier periods of the growing season especially for the first tomato (Fig. 4a) and rape (Fig. 4b); second rape (Fig. 4d) crops when low NO3–N leaching was expected.
The narrow C:N ratios of high and low N manures may not necessarily mean that they readily release mineralized N upon microbial degradation (Mtambanengwe et al. 1998). The materials constituting high N manure contain high levels of reactive phenols and tannins (Jassal et al. 2011), which polymerize with a range of amino acids (containing N) from microbially degraded crude proteins in applied manures to generate complexes, which are resistant to enzymatic decomposition by micro organisms in wetland soil systems (Zingore et al. 2008). This resulted in slow release of mineralized N into the soil solution recorded during the early stages of this study.
The first season after application of high and low N manures may have encouraged a rapid growth of microbial biomass due to an abundance of organic substrate (Ouedraogo et al. 2007), which placed a heavy demand for N on the limited amounts of mineralized N from slowly decomposing manure in soil. The active uptake and assimilation of the limited reserves of mineralized N by the microbiotica in manure fertilized plots is suspected to have weakened the superior potential of the high N manure over low N manure to supply mineralized N in soil observed during the first 42 days after application of the two types of cattle manure. As result, the differences between the concentrations of mineral N in soils subjected to low and high N manure applications (Figs. 3, 4) were comparatively small before 42 days after planting the tomato and rape crops.
Nitrous oxide fluxes from soil
The fluxes of N2O at the time of gas sampling for the period between 21 September 2007 and 9 November 2008 are given in Fig. 5. Study results show that N2O fluxes following high N manure applications were significantly higher (p < 0.05) when compared with fluxes after application of low N manure. Emissions of N2O from wetland soil amended with cattle manure increased considerably with increasing content of N in the applied manure. Higher N2O emissions were recorded in the first gas samples collected from vegetable plots amended with applications of 30 Mg/ha high and low N manure, which was applied a week before planting the first tomato crop. In high and low N manure applications, elevated N2O fluxes persisted throughout the 98 and 84-day period for tomato and rape crops, respectively, when compared with emissions on the control plots. The substitution of 15 and 30 Mg low N manure/ha applied once in the four cropping events by the same rates of high N manure increased N2O fluxes in soil by 41 % (2.5 g/ha/day) and 50 % (3.6 g/ha/day), respectively.
N2O emissions from wetland soil during the September 2007 to November 2008 period followed a pattern that comparatively matched the rainfall events at Dufuya (Figs. 2, 5). For the first tomato and rape and second rape crops the rise in the N2O emission coincided with wet weather spells (Fig. 5a, b). In a study on the effects of temperature, water content and N fertilization on emissions of nitrous oxide by soils, Smith et al. (1997) confirmed that exponential relationships between N2O flux and both water-filled pore space and temperature are only observed when soil mineral N is not limiting. Lower fluxes of N2O emission were recorded in the second tomato, a crop which grew under dry weather conditions of the 2008 April to July winter season (Fig. 2). Addition of manure to wetland soil enhances the potential for denitrification and increased emissions of N2O gas (Ma et al. 2007) through a general stimulation of microbial respiration, causing rapid oxygen consumption and consequently an increase of anaerobic conditions for accelerated denitrification (Johnson et al. 2005; Jassal et al. 2011). While C from manure stimulates microbial respiration, water in a wetland soil limits O2 diffusion in soil (Van der Meer 2008; Snyder et al. 2009). Flooded soils have aerobic and anaerobic zones (Johnson et al. 2005), allowing both nitrification and denitrification to take place simultaneously. In fact, Snyder et al. (2009) concluded that N2O emissions to the atmosphere are highest where conditions are sub-optimal for nitrification and denitrification. Since the first process produces the substrate for the second, N losses from applied cattle manure can be very high when the two processes are associated. As much as 60–70 % of fertilizer N applied to wetland crop may be volatilized as oxides of N (Munoz et al. 2010).
The higher emission of N2O in plots amended with high N manure when compared with emissions on plots subjected to low N manure applications was attributed to the case of increased capacity of the wetland soil that received high N manure to supply mineralized N beyond soil microbial requirements (Mosier and Kroetze 1999), plant uptake and precipitation in lingo-protein complexes (Saggar 2010). The result was a net balance of NO3–N in wetland soil solution, which acted as a substrate for the N2O-releasing denitrification process (Ma et al. 2007). This significantly enhanced potential of wetland soil subjected to high N manure applications to release N2O to the atmosphere.
Estimated total N lost in N2O emission following application of high and low N manure
There were significant treatment differences (p < 0.05) on total N lost through emission of N2O during the growing period of tomato and rape (Tables 3, 4). Estimated total N lost through N2O emissions on plots subjected to 15 and 30 Mg high and low N manure applications were 26 and 85 % above the emissions recorded on control plots on average, respectively. When 15 and 30 Mg of low N manure/ha were replaced by the same rates of high N manure total N lost through N2O emission increased by 68 and 51 %, respectively.
Mean ranges of total N lost as N2O from manure fertilized plots (0.21–0.74 kg N/ha, Tables 3, 4) may appear small, but at global level, cumulative quantities of N lost as N2O are significant because N2O has a global warming potential of 270–320 times compared to CO2 (Ma et al. 2007; Snyder et al. 2009; Smith 2012). In addition, N2O can last approximately 100–150 years (Grant and Beer 2008; Munoz et al. 2010; Saggar 2010) in the atmosphere, so that a kg of N2O emitted is potentially more damaging than the same unit of CO2. The relatively small amounts of total N lost per unit area as N2O may explain why N2O is responsible for only 4–6 % of the greenhouse effect compared to 50 % for CO2 (Smith 2012).
The applications of lower rate of high and low N manures were followed by lower emissions of N2O, a result that is favorable to the objective of lowering the contribution of agricultural sources to the global greenhouse gas emissions. However, the same practice significantly reduced dry matter yield (vegetable yield) (Tables 5, 6). Smallholder farmers are engaged in wetland vegetable farming to obtain higher yields for increased vegetable sales when compared with dry land cropping. In this situation, there is a conflict of local smallholder farmer goals and the global objective of reducing emissions from agricultural sources. The use of low N cattle manure from the adjacent Vungu commercial farming area might be a way to reduce emissions of N2O from wetland vegetable production at Dufuya.
Uptake following application of high and low N manure
Results presented in Tables 5 and 6 clearly show that there were significant (p < 0.05) manure quality treatment effects on the dry matter yield and uptake of N by all four crops in the study. Fertilization of the vegetable crops using 15 Mg and 30 Mg/ha of high N manure significantly (p < 0.05) increased N uptake by 63 and 106 kg N/ha above that recorded for the control plots, respectively. Crops in soil subjected to 15 Mg and 30 Mg/ha applications of low N manure assimilated 43 and 94 kg N/ha in excess of the N uptake by plants in unamended soil. The use of 15 Mg and 30 Mg/ha high N manure instead of low N manure increased N uptake by 22 and 21 kg/ha, respectively.
The uptake of N by crops may act as a bio-sink for N that can otherwise be exposed to denitrification (Billy et al. 2010). The use of low N manure reduced N uptake by all test crops. This was attributed to the fact that the content of N in the smallholder manure was more than double the content of N in commercial farm manure. The elevated content of N in the smallholder manure increased its capacity to supply mineralized N for crop biomass accumulation, which in turn increased uptake of N from the wetland soil system. The N content and C:N ratio of applied organic residues are important for N immobilization processes after incorporation, where microbial biomass acts as a sink for N (Nyamangara and Nyagumbo 2010).
Total N lost in N2O emission per unit dry matter
Tables 5 and 6 show N lost in N2O emission per unit of harvested dry matter yield. When the application rates of high and low N manure were increased from 15 to 30 Mg/ha the emissions of N2O per unit harvested dry matter of rape and tomato significantly decreased (p < 0.05). The estimated loss of N in N2O emissions decreased by 0.01–0.03 and 0.01–0.06 kg N–N2O per Mg of harvested dry matter when high and low N manure application rates were increased from 15 to 30 Mg/ha, respectively. Nitrous oxide emission losses per unit harvested dry matter of tomato crop were significantly (p < 0.05) higher in the unamended plots than on manure fertilized plots (Tables 5, 6). However, losses of N in N2O emissions per unit harvested dry matter from the control plots under rape crop were generally lower when compared with the losses from manure fertilized plots. Study results show that there were detectable treatment separations on the basis of N content in cattle manure (manure quality) in losses N per unit harvested dry matter yield after emissions of N2O (p < 0.05) from the plots. When 15 Mg of low N manure/ha was substituted with 15 Mg/ha of high N manure, the loss of N in emissions of N2O from plots decreased significantly (p < 0.05) by 0.01–0.10 kg N per unit harvested dry matter.
Increased dry matter accumulations on plots subjected to higher cattle manure applications were followed by higher uptake of N from the applied fertilizers. Consequently, plots that were amended with higher rates of manure applications effectively sequestrated N that may be exposed to denitrification and the associated emissions of N2O. This implies that when agronomic practices are improved through manure applications, the loss of N in N2O emissions may significantly decrease.
Correlations between measured variables
Figures 6 and 7 show the regression analyses indicating the direct and indirect effects of soil factors on N2O emissions following application of high and low N manures. The regression analysis has shown that the relationships between soil moisture and N2O emissions were significantly (p < 0.05) correlated with r 2 values ranging from 0.44 to 0.59. The direct proportional relationship between soil moisture and emissions of N2O was recorded during the growing period of the first tomato and second rape crops only (Figs. 6, 7a, b).
A direct proportional relationship was recorded between NH4–N in soil and emissions of N2O from soil with r 2 values ranging from 0.40 to 0.73. The concentration of NO3–N in soil subjected to applications of high and low N manure was significantly correlated with the emissions of N2O from soil with coefficients of determination ranging from 0.41 to 0.73. The r 2 values for the relationships between NH4–N, NO3–N in soil and emissions of N2O were comparatively similar. However, the regression analysis has shown that the r 2 values for the relationships between soil moisture content and N2O emissions (r 2 of 0.44–0.59, Figs. 6a, b, 7a, b) were smaller than the r 2 values (r 2 of 0.40–0.73 for NH4–N, 0.41–0.73 for NO3–N) for the relationships between NH4–N, NO3–N and N2O emissions from soil.
The influence of soil moisture on variability found in emissions of N2O after application of high and low N manures was only recorded in the first tomato and second rape crops (Figs. 6, 7a, b). The manure organic matter decomposition processes are oxidative and proceed only in the presence of water at least up to field capacity moisture content. The vegetative periods of the first tomato and second rape crops occurred over both dry (September 2007 and 2008) and wet summer seasons (after October 2007 and 2008). This introduced a change in wetland hydrology that influenced dynamics in the N mineralization processes over the cropping seasons in one cropping event. Regression analysis conducted on NH4–N and NO3–N in soil and N2O emissions (Figs. 6, 7) showed the influence of NH4–N and NO3–N concentrations in soil on N2O emissions were comparatively similar. Both processes of nitrification of NH4–N and denitrification of NO3–N are thought to contribute immensely to the emissions of N2O although the later has been suggested to play a bigger role in the emissions (Ma et al. 2007). The r 2 values for the relationships between soil moisture content and N2O emissions (r 2 of 0.44–0.59, Figs. 6a, b, 7a, b) were smaller than the r 2 values (0.40–0.73 for NH4–N, 0.41–0.73 for NO3–N) for the relationships between NH4–N, NO3–N and N2O emissions from soil. This implies that the concentration of mineral N in after the decomposition of cattle manure is a stronger predictor of N2O emissions in manure fertilized soils when compared with the content of soil moisture.
Conclusions
Results from the current study demonstrate that the loss of N in N2O emissions from wetland soil subjected to cattle manure applications constitutes an important nutrient flux, and the magnitude of the losses depended upon application rate and content of N (manure quality) in applied manure. Given that N2O in agricultural soil is produced predominantly through the microbial transformations of inorganic N, the potential to produce and emit N2O increases with the increasing availability of N and consequently the concentration of N in applied manure. It can generally be concluded that mineral N concentrations in soil; dry matter yield of rape and tomato; N uptake and emissions of N2O from wetland soil amended with cattle manure increases with increasing content of N in applied manure (manure quality) and rate of application. The loss of N in emissions of N2O expressed per unit mass of harvested dry matter yield of rape and tomato crops decreases significantly with increasing manure application, dry matter yield and N uptake. Improved agronomic practices for increased crop productivity can be used as a mitigation factor for reducing the contribution of agriculture in the global emissions of N2O.
Abbreviations
- C:
-
N Carbon to nitrogen ratio
- IPCC:
-
Intergovernmental Panel for Climate Change
- GHG:
-
Greenhouse gas
- FAO:
-
Food and Agriculture Organization
- AGRITEX:
-
Department of Agricultural Technical and Extension Services
References
Billy C, Billen G, Sebilo M, Birgand F, Tournebize J (2010) N isotopic composition of leached nitrate and soil organic matter as an indicator of denitrification in a sloping drained agricultural plot and adjacent uncultivated riparian buffer strips. Soil Biol Biochem 42:108–117
Black GG, Hartge KH (1986) Bulk density. Methods of soil analysis. Part 3. ASA, Madison, pp 363–375
Bouyoucos GJ (1965) Hydrometer method improved for making particle size analysis of soils. Agronomy J 27:738–741
Bremner JM (1996) N total. In: Sparks DL et al (eds) Methods of soil analysis: part 3. chemical methods, vol 5. Soil Science Society of America, Book series Soil Science Society of America, Inc., American Society of Agronomy, Inc, Madison, pp 1085–1121
Bremner JM, Mulvaney CS (1982) N-total. In: Page AL (ed) Methods of soil analysis. Agronomy series no. 9, part 2. American Society of Agronomy, Madison, pp 595–622
Conrad R, Seiler W, Bunse G (1983) Factors influencing the loss of fertilizer N into the atmosphere as N2O. Geophys Res 88:6709–6718
De Lannoy G (2001) Vegetables: pumpkins. In: Raemaekers RH (ed) Crop production in tropical Africa. Director-General for International Cooperation (DGIC), Brussels, pp 459–461
Dobbie KE, McTaggart IP, Smith KA (1999) Nitrous oxide emissions from intensive agricultural systems. Variations between crops and seasons. Key driving variables and mean emissions factor. J Geophys Res Atoms 104:26891–26899
Food and Agriculture Organization (1988) FAO/UNESCO Soil Map of the World, Revised Legend, with corrections and updates. World Soil Resources Report 60, FAO, Rome. Reprinted with updates as technical paper 20, ISRC. Wageningen, The Netherlands, p 140
Galle B, Weslien P, Samuelsson J, Klemedtsson AK, Klemedtsson I (2003) An automatic field chamber system based on FTIR GC analysis, applied in measurements of N2O and CO2 emission from an organic soil. Eur J Soil Sci 54:234–248
GenStat Discovery Edition 3 (2003) GenStat for windows (7th Edition) introduction. VSN International, Oxford
Grant T, Beer T (2008) Life cycle assessment of greenhouse gas emissions from irrigated maize and their significance in the value chain. Aust J Exp Agric 48:375–381
Grenon F, Bradley RL, Titus BD (2004) Temperature sensitivity of mineral N transformation rates, and heterotrophic nitrification: possible factors controlling the post-disturbance mineral N flush in forest floors. 2004. Soil Biol Biochem 36:165–1474
Groot JCJ, Rossing WAH, Lantinga EA (2006) Evolution of farm management, N efficiency and economic performance on Dutch dairy farms reducing external inputs. Livest Sci 100:99–110
Hickman JE, Havlikova M, Kroeze C, Palm CA (2011) Current and future nitrous oxide emissions from African agriculture. Environ Sustain 3:370–378
Holland EA, Robertson GP, Greenberg J, Groffman P, Boone R, Gosz J (1999) Soil CO2, N2O and CH4 exchange. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford, New York, pp 185–201
Hutchinson GL, Livingston GP (1993) Use of chamber systems to measure trace gas fluxes, p. 63–78. In: Harper LA et al (eds) Agroecosystem effects on radiatively important trace gases and global climate change. ASA special publication 55. ASA, CSSA and SSSA, Madison, pp 234–241
IPCC (2001) Technical summary. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA (eds) Climate change. The scientific basis. Contribution of working group 1 of the international panel on climate change. Cambridge University Press, Cambridge, pp 345–356
Jassal RS, Black TA, Roy R, Ethier G (2011) Effect of N fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 162:182–186
Johnson JMF, Roycosky DC, Almaras RR, Sauer TJ, Venterea RT, Dell CJ (2005) Greenhouse gas contribution and mitigation potential of agriculture in the central USA. Soil Till Res 83:73–94
Kaiser EA, Munch JC, Heinemeyer O (1996) Importance of soil cover box area for the determination of N2O emissions from arable soils. Plant Soil 18:185–192
Kroetze C, Aerts R, Van Dam D, Van Der Hoek K, Hofschreuder P, Hoosbeek M, de Klein J, Kros H, Van Oene H, Oenema O, Tietema A, Van Der Veeren R, de Vries W (2003) Uncertainities in the fate of N. An overview of sources of uncertainty illustrated with a Dutch case study. Nutr Cycl Agroecosyst 66:43–69
Lin J, Hu RJ, Zhao JR, LeiLei MS (2011) Nitrous oxide emissions from rape field as affected by N fertilizer management: a case study in Central China. Atmos Environ 45:1775–1779
Ma WK, Schautz A, Fishback LE, Bedard-Haughn A, Farrell RE, Siciliano SD (2007) Assessing the potential of ammonia oxidizing bacteria to produce nitrous oxide in soils of high arctic lowland ecosystem on Devon Island, Canada. Soil Biol Biochem 39:2001–2013
Mafongoya PL, Nair PKR, Dzowela BH (1998) Mineralization of N from decomposing leaves of multipurpose trees as affected by their chemical composition. Biol Fertil Soils 27:143–148
Mapfumo P, Mtambanengwe F, Vanlauwe B (2007) Organic matter quality and management effects on enrichment of soil organic matter fractions in contrasting soils in Zimbabwe. Plant Soil 296:137–150
Markewich HA, Pell AN, Mbugua DM, Cherney DJR, Es HM, van Lehmann J, Robertson JB (2010) Effects of storage methods on chemical composition of manure and manure decomposition in soil in small-scale Kenyan systems. Agric Ecosyst Environ 139:134–141
Masvaya EN, Nyamangara J, Nyawasha RW, Zingore S, Delve RJ, Giller KE (2011) Effect of farmer management strategies on spatial variability of soil fertility and crop nutrient uptake in contrasting agro-ecological zones in Zimbabwe. Nutr Cycl Agroecosyst 88:111–120
Materechera SA (2010) Utilization and management practices of animal manure for replenishing soil fertility among small-scale crop farmers in semi-arid farming districts of the North West Province, South Africa. Nutr Cycl Agroecosyst 87:415–428
Mathias AD, Blackmer AM, Bremmer JM (1980) A simple chamber technique for field measurement of emissions of nitrous oxide from soils. J Environ Qual 9:251–259
Meyer CP, Galbally IE, Wang Y, Weeks IA, Jamie I, Griffith DWT (2001) Two automatic chamber techniques for measuring soil atmosphre exchanges of trace gases and results of their use in the Oasis field experiment. CSIRO Atmosphere Research Technical Paper No. 51, pp 1–33
Ministry of Environment and Natural Resources Management. 2010. Annual Report. Harare
Mosier AR, Kroetze C (1999) Contributions of agroecosystems to the global atmospheric N2O budget. In: Desjardins RL, Keng JC, Haugen-Kozyra K (eds) Proceedings of the International Workshop of reducing nitrous oxide emissions from Agroecosystems, Banff, Alberta, March 3–5. Agriculture and Agri-Food Canada, Research Branch, Alberta Agriculture, Food and Rural Development, Conservation and Development Branch, pp 3–15
Mosier AR, Mack L (1980) Gas chromatographic system for precise and rapid analysis of nitrous oxide. Soil Sci Soc Am J 44:1121–1123
Mosier A, Kroeze C, Nevison C, Osnema O, Seitzinger S, van Cleemput O (2003) Closing the N2O budget: nitrous oxide emissions through the agricultural N cycle. Nutr Cycl Agroecosyst 52:225–248
Mtambanengwe F, Chivaura-Mususa C, Kirchmann H (1998) In: Woomer PL, Swift MJ (eds) Assessment of plant litter quality related short-term carbon and N mineralization in soil. The biological management of tropical soil fertility. Wiley, New York, pp 81–116
Mugandani R, Wuta M, Makarau A, Chipindu B (2012) Re-classification of agroecological regions of Zimbabwe in conformity with climate variability and change. Afr Crop Sci J 20:361–369
Munoz C, Paulino L, Monreal C, Zagal E (2010) Greenhouse gas (CO2 and N2O) emissions from soils: a review. Chil J Agric Res 70:485–497
Nelson DW, Sommers LE (1982) Methods of soil analysis. In: Page AL (eds) Total C, organic C and organic matter. Agronomy series No. 9, Part 2. The Agronomy Society of America, Madison, pp 539–579
Nelson DW, Sommers LE (1986) In: Page AL (ed) Methods of determination of total organic carbon (TOC) in soils and sediments. Total C, organic C and organic matter. Ecological Risk Assessment Centre, Office of Research and Development, US. Environmental Protection Agency, Las Vegas, pp 456–461
Nyamangara J, Nyagumbo I (2010) Interactive effects of selected nutrient resources and tied-ridging on plant growth performance in a semi-arid smallholder farming environment in central Zimbabwe. Nutr Cycl Agroecosyst 88:103–109
Nyamapfene KW (1991) Soils of Zimbabwe. Nehanda Publishers (Pvt) Ltd, Harare, pp 75–79
Ouedraogo E, Stroosnijder L, Mando A, Brussaard L, Zougmoré R (2007) Agroecological analysis and economic benefit of organic resources and fertiliser in till and no-till sorghum production after a 6-year fallow in semi-arid West Africa. Nutr Cycl Agroecosyst 77:245–256
Owen R, Verbeek K, Jackson J, Steenhuis D (1995) Water management, cropping and soil potentials for smallholder farming in the wetlands. University of Zimbabwe, Harare
Robertson GP, Wedin D, Groffman PM, Blair JM, Holland EA, Nadelhoffer KJ, Haris D (1999) In: Robertson GP et al (eds) Soil carbon and N availability: N mineralization, nitrification, and soil respiration potentials. Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 258–271
Saggar S (2010) Special issue: estimation of nitrous oxide emission from ecosystems and its mitigation technologies. Agric Ecosyst Environ 136:189–365
Silva RG, Holub SM, Jorgensen EE, Ashanuzzaman ANM (2005) Indicators of nitrate leaching loss under different land use of clayey and sandy soils in southern Oklahoma. Agric Ecosyst Environ 109:346–359
Smith P (2012) Agricultural greenhouse gas mitigation potential globally, in Europe and in the UK: what have we learnt in the last 20 years? Glob Change Biol 18:35–43
Smith KA, McTaggart IP, Tsuruta H (1997) Emissions of N2O and NO associated with N fertilization in intensive agriculture and the potential for mitigation. Soil Use Manag 13:296–304
Snyder CS, Bruulsema TW, Jensen TL, Fixen PE (2009) Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric Ecosyst Environ 133:247–266
Soil Survey Staff (1992) Keys to the soil taxonomy, fifth edition, Blacksbury, Virginia. SMSS Tech Monogr Number 19:541
Stevenson FJ (1982) Methods of soil analysis. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Agronomy no. 9, vol 2. American Society of Agronomy, Madison, pp 625–641
Van der Meer HC (2008) Optimizing manure management for greenhouse outcomes. Aust J Exp Agric 48:38–45
Van Der Salm C, Dolfing J, Heinen M, Velthof GL (2006) Estimation of N losses via denitrification from heavy clay soil under grass. Agric Ecosyst Environ 119:311–319
Vasileiadou E, Heimeriks G, Petersen AC (2011) Exploring the impact of the IPCC assessment reports on science. Environ Sci Policy 14:1052–1061
Vincent V, Thomas RG (1960) An agricultural survey of southern Rhodesia, part I agro-ecological survey. Government Printers, Salisbury
Whitlow JR (1985) Research on dambos. Zimbabwe Agric J 82(2):59–66
Wuta M, Nyamugafata P (2012) Management of cattle and goat manure in Wedza smallholder farming area, Zimbabwe. Afr J Agric Res 7(26):3853–3859
Yates TT, Si BC, Farrell RE, Pennock DJ (2006) Probability distribution and spatial dependence of nitrous oxide emission: temporal change in hummocky terrain. SSSA 70:753–762
Zingore S, González-Estrada E, Delve RJ, Herrero M, Dimes JP, Giller KE (2008) An integrated evaluation of strategies for enhancing productivity and profitability of resource-constrained smallholder farms in Zimbabwe. Agric Syst 101:57–68
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Masaka, J., Nyamangara, J. & Wuta, M. Nitrous oxide emissions from wetland soil amended with two types of cattle manure. Int J Recycl Org Waste Agricult 5, 125–140 (2016). https://doi.org/10.1007/s40093-016-0123-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-016-0123-9