Abstract
Mineral carbonation is a promising CO2 sequestration strategy that offers a long-lasting and environmentally safe solution. In this study, the effect of pH, salinity and particle size in the mineral carbonation process was investigated. Ultramafic–mafic rock samples were collected from different ophiolite rock sampling sites in Luzon Island, Philippines, and these were used in mineral carbonation reaction. Dissolution experiments were conducted by exposing powdered rock samples in suspensions sparged with CO2 for 60 days at ambient conditions (25 °C and 1 bar). Carbonation reactions were observed at various pH conditions (4, 6, and 10) and particle sizes (62–125 and 250–420 μm). In separate experiments, the effects of pH and salinity were studied in experimental set-ups containing 5 % MgCl2 maintained at low and high pH. Inductively coupled plasma-mass spectrometry (ICP-MS) was used to monitor concentrations of metals that could participate in the mineralization reaction (Mg, Al, Ca, and Fe) during exposure to CO2. X-ray diffraction (XRD) analysis was used to confirm the formation of carbonate minerals. Results indicate an enhancement in the carbonation process upon varying pH and salinity of the system, while there is a negligible difference in the mineral carbonation reaction at the range of particle sizes used in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mineral carbonation is a very promising CO2 sequestration method and is already considered viable in different types of geological frameworks. Mineral carbonation has many advantages due to the fact that (1) there are abundant sources in the environment of Ca and Mg, which are needed to store excess CO2 as carbonate minerals [17]; (2) it reduces the risks associated with pressurized geologic injection of CO2, such as possible leakage, contamination of potable ground water, and other unpredicted seismo-geological incidents [3]; and (3) the end-products of this process do not pose environmental threats [16].
The reactions involved in the mineral carbonation process in rock samples are: first (1) conversion of CO2(g) into CO3 2− (aq); then (2) dissolution of minerals to release the metal cations (Ca2+ and Mg2+); finally (3) the combination of carbonate anion and metal cation to form the carbonate minerals [24, 25]. In these reactions, the mineral dissolution reaction is the rate-determining step [35], thus enhancing mineral dissolution reaction will also yield faster mineralization.
Flow injection pilot tests and other feasibility studies have recognized the full potential of basaltic deposits for CO2 storage [4, 5, 22, 33, 36]. Other rock formations such as the Samail Ophiolite of the Sultanate of Oman have been considered in several studies for natural CO2 sequestration sites [14, 26]. More rock formations found in an archipelagic settings has also been considered [2]. These geological frameworks have a high CO2 sequestration capacity through mineral fixation and solubility trapping [7]. However, though these geological frameworks have significant potential, physical and chemical activation treatments are still necessary to facilitate in stepping up the reaction rates of metal ion dissolution for ensuing carbonation [1]. These interventions vary from heat or steam treatments, to leaching agents and complexing agent usages [1, 13, 21, 29]. One prospect to include in considering an injection host is the different types of geological frameworks with nearby alkaline water reservoirs.
The method of CO2 injection into serpentinitic aquifers poses a feasible method in improving the interaction of CO2 with dissolved metal cations. Geochemical data on spring waters involving serpentinitic rocks and ultramafic rocks have shown this potential [7]. Alkaline springs are associated with ophiolitic rocks and assumed to equilibrate with atmospheric CO2 and eventually form calcite, brucite and aragonite [6, 30, 34]. Salinity and pH are important features of these alkaline springs and would prove beneficial in the dissolution step. Salinity and pH are important factors in carbon mineralization reactions; the latter parameter is important evidently because H+ concentration influence formation of HCO3 − (dissolution of CO2 in water), which will then react to the available metals in the system [8, 28]. Changes in pH would be observed once CO2 is injected into reaction mixtures such as brine solutions, which will in turn influence the uptake of divalent cations, and eventually promote carbonate formation [18, 19]. Salinity is also a parameter to study since an increase in salinity might result to low dissolution of CO2 [8, 32]. The presence of nearby alkaline springs would eliminate the required chemical interventions in the dissolution of metal ions from the host minerals. Experimental studies on the assessment of carbonation reactions using these types of rocks in the presence of alkaline solutions are quite limited [20].
Particle size also plays an important role in mineral dissolution reaction because, theoretically, a smaller particle size will result in faster dissolution and will increase the availability of divalent metal cations. On the contrary, reducing the particle size will require additional energy in the over-all process, and the enhancement may or may not be sufficient to compensate for the additional energy input [10, 12].
Small-scale or laboratory scale investigations must constantly be conducted to continuously develop energy-conscious and economically feasible CO2 sequestration processes on carbonation reactions. In this study, suspensions of different ophiolitic rock samples were prepared to evaluate different experimental conditions operating at minimal energy input (ambient pressure and temperature conditions) on the formation of carbonates. Gaseous CO2 was directly bubbled into the suspensions to produce CO2-sparged aqueous solutions. Salinity and pH were considered to resemble the alkaline spring waters in natural settings. A parametric study was done on these two factors as part of artificial enhancement pre-treatment steps on carbonation rates. Observed alterations on the mineral surfaces (in all suspensions) are attributed to carbonate formation at different pH and salinity of rock suspensions as well as particle sizes of the rock samples in CO2-sparged aqueous solutions.
Materials and methods
Chemicals
All chemicals and reagents used in this study were of analytical reagent grade unless otherwise specified. High purity CO2 (99.9 %, Linde Phils.) was used in the carbonation reaction, Hydrochloric acid (RCI Labscan, Thailand) and sodium hydroxide (HiMedia Laboratories, India) were used to adjust the pH of the system. Magnesium chloride (HiMedia Laboratories, India) was used to prepare the required salinity for aqueous carbonation experiments. All dilutions and solution preparations for the ICP-MS analysis were done using ultrapure water prepared using a Millipore Direct-Q5, Ultrapure Water Purification System (18.2 M cm resistivity, Merck Millipore). The rock powder was decanted then air dried prior to XRD analysis.
Sample collection initial characterization
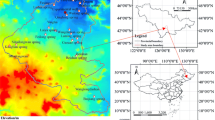
Ophiolitic rock samples were collected from different locations in Luzon as shown in Fig. 1; namely Angat Ophiolites (AO), Zambales Ophiolite Complex (ZOC) and Camarines Norte Ophiolite Complex (CNOC). The obtained rock samples were submitted to the Mines and Geosciences Bureau, Petrolab, for thin-section petrographic analysis.
The rock samples were crushed and powdered prior to carbon mineralization experiments. Laboratory mesh sieves were used for the particle size-controlled experiments. Fractions of fine (62–125 μm) and (250–420 μm) coarse particle sizes were chosen in this study.
Mineral carbonation experiments
The experimental design for the carbonation experiment was based on a simple direct aqueous carbonation method, wherein water and grounded rock suspension was supplied with CO2 to facilitate both the mineral dissolution reactions as well as the formation of HCO3 − species. Baseline carbonation studies were previously conducted [20] on the mineral rock samples by sparging the powdered rock-water mixture with CO2, under natural pH and ambient conditions (1 bar and 298 K). In addition, mineral carbonation experiments were also conducted under high and low pH (pH 13 and 1, respectively). To further study the effect of pH in mineral carbonation reactions, carbonation experiments were conducted under intermediate pH (pH 4, 6 and 10). Increase or decrease in the concentrations of Mg, Al, Ca and Fe were monitored to determine the dissolution of the rock samples at different pH values.
Mineral carbonation experiments were done by adding 10 mL water to 10 g powdered rock samples in 20 mL vials and sparging (5 mL/min) the suspensions with CO2 for 60 days.
pH-only and pH-salinity experiments
The effect of pH was studied by maintaining the carbonation reaction vials at pH 4, 6, and 10. The effects of both pH and salinity were observed using; (1) a high pH condition (pH ~13) with 5 % MgCl2, and (2) a low pH condition (pH ~1) also with 5 % MgCl2, these were compared against suspensions wherein the pH and salinity were not modified.
Bubbling of CO2 into suspensions was also conducted for 60 days. The suspensions were prepared as follows: (1) unaltered condition (no modification in pH and salinity of the suspensions); (2) low pH with 5 % MgCl2; and (3) high pH with 5 % MgCl2, these were all compared with the X-ray diffraction pattern of the untreated rock sample (i.e., not purged with CO2). The water used for the “unaltered” set-up is taken from the streams near the sampling site of the rock samples. The pH and salinity were not modified.
Grain size range used for pH, and pH-salinity experiments was 62–420 µm.
Grain size only experiments
For the particle size-controlled experimental batch, carbonation experiments were done on fine (particle size: 62–125 μm) and coarse (particle size: 250–420 μm) rock samples. No quantification measurements were conducted to determine the amount of CO2 uptake in all experiments.
Instrumentation
The CO2–water–rock interaction that occurred upon bubbling the powdered rock samples with CO2 was studied by monitoring the metal concentration in the aqueous part of the system as well as the change in the mineral composition of the rock samples. An Agilent 7500cx inductively coupled plasma-mass spectrometry (ICP-MS) was used to quantify the metal content of the samples. Shimadzu MAXima_X XRD-7000 X-ray diffractometer (XRD) was used to monitor the change in mineral composition of the rock samples.
Results and discussion
Ophiolitic rock compositions
Mineral composition of each of the ophiolitic rock samples used in the investigation is summarized in Table 1. Samples utilized are mostly of magnesium and calcium bearing minerals. Angat ophiolites are composed mainly of plagioclase (60 %) and considered as an Altered Diabase rock. Zambales ophiolites are composed mostly of serpentine (73 %) and identified as Serpentinite rock. Camarines Norte ophiolites are a mixture of Enstatite (38 %), Augite (30 %) and Tremolite (20 %). It was classified as an Altered Gabbro rock.
Carbonate formation under different pH only conditions
Availability of Ca2+ and Mg2+ ions is one of the controlling factors for the development of in situ CO2 mineralization [30]. Dissolution of these metals into CO2-sparged solutions would be easily available for CO2 consumption [26]. Ophiolitic rock samples are rich with these divalent cations, thus, the decrease of metal concentrations in the purged suspensions indicates elemental scavenging by CO2. Results from ICP-MS analyses, as seen in Fig. 2, showed a distinctive trend wherein an increase in the metal concentration in the aqueous layer was observable in the 15th and 30th day of CO2 exposure, which could be accounted for the dissolution of the minerals from the rock samples. As expected, Mg2+ ions were detected prominently indicating the possibility of extracted Mg2+ ions dissolved into the aqueous layer of ZOC suspensions. As observed from petrographic analyses, Mg2+ ions are prominent in ZOC suspensions mainly because of the intrinsic mineral composition. At all pH conditions, Mg2+ ions showed increased concentrations on the 15th day of bubbling CO2 into the suspensions. As can be seen in Fig. 3, the intensity of the carbonate mineral peak is not as significant compared to the original ‘serpentine peaks’ (for the carbonation experiment carried under ambient conditions). And for the “enhanced” carbonation set-up, the composition is still not entirely changed into newly formed carbonate minerals as the presence of the original peaks attributed to the original mineral components of the rock samples are still evident. The “enhanced” carbonation set-up is a suspension that is considered as more favorable condition for carbonation reaction. Thus, formation of MgCO3 is not easily formed, even though the Mg2+ ions are dissolved in the suspensions and available for formation reactions. This indicates that MgCO3 mineral, in an extended carbonation period, may eventually form in these rocks. The formation of MgCO3 might have been suppressed in early reaction times by the presence of the MgCl2 in the suspensions. A need to investigate the influence of Mg2+/Ca2+ molar ratios in the MgCO3 and CaCO3 formation should be considered in future studies.
X-ray diffraction patterns of rock samples after 60 days of subjecting to mineral carbonation experiments. a Untreated: not subjected to carbonation experiments; b baseline: ambient conditions, no modification in pH and salinity; c low pH, 5 % MgCl2; d high pH, 5 % MgCl2. Peak at 2.899 is assigned to hydromagnesite while peak at 3.035 is assigned to calcite. Y-axes are all on the same scale
The identification of the source of Mg2+ ions among the ophiolite rocks which contribute to the new MgCO3 formed is not easily discerned from the carbonation experiments, since the rock samples are already composed of a mixture of minerals. Based on the dissolution rates of enstatite, augite and serpentine, with respective log K: −9.02, −6.82, and −5.70 values [27], it can be inferred that serpentine undergoes faster dissolution than enstatite and augite, thus there are more available Mg2+ ions from serpentine than the other minerals.
Aluminum, iron and calcium ions showed a similar trend in suspensions of Angat ophiolites indicating that these ions may be easily available for new mineral formation during carbonation process. A decrease in the metal concentrations (Mg, Al, Ca, and Fe) was observed during the 45th and 60th day of bubbling which could indicate the consumption of CO2 and mineralization could have taken place. For the pH-controlled carbonation vessels, the process of mineral dissolution (of the original mineral components of the rock samples) and re-precipitation (formation of carbonate minerals) were observed by monitoring the changes in the concentration of metals (that participate in the reaction) in the aqueous component of the rock-water-CO2 mixture. With these observations, carbonate formation or new mineral formation on Zambales ophiolites are still considered favorable at working pH (pH = 4, 6 and 10) compared with Angat and Camarines Norte ophiolites.
Carbonate formation under different pH and salinity conditions
Another experimental batch was prepared to investigate the effects of both pH and salinity on the mineral carbonation reaction. Salinity is considered to reduce solubility trapping due to reduction in solubility of CO2 and change in the partial molar volume of water [11]. The metal concentrations were monitored in this set-up (Fig. 2) and the carbonate phases resulting from the carbonation procedure were visible from the XRD spectra. Significant changes on the rock surfaces were observed as seen from Fig. 3. An observable change in the mineral content in the powdered rock sample is the appearance of peak at 2θ = 29.42. This corresponds to a d-spacing = 3.035, which is a characteristic of the carbonate mineral, Calcite [CaCO3]. This peak was evident in all the samples subjected to mineral carbonation. The difference in the intensity is attributed to the relative amount of the formed mineral in the powdered rock sample. Using a similar rationale for the new MgCO3 mineral formation, it would be difficult to distinctly identify the source of Ca2+ ions for the formation of CaCO3. Between the two minerals, tremolite and plagioclase, with respective log K: −8.40, and −7.5 values [27], dissolution of plagioclase would be faster, and thus could be the easier source of Ca2+ ions.
Aside from the appearance of characteristic peaks of carbonate based minerals, the decrease in the intensity of peak at 2θ ~12.1 (which corresponds to d-spacing ~7.31) could be an evidence of the dissolution of serpentine group of minerals (i.e., lizardite [Mg3Si2O5(OH)4]) which is one of the original mineral component of the rock samples. An additional peak at 2θ = 30.84 (d-spacing = 2.899) appeared in the sample subjected to high pH with 5 % MgCl2, this could be assigned as the characteristic peak of hydromagnesite [Mg5(CO3)4(OH)2·4H2O], which is very probable due to the high alkalinity at the given experimental conditions. Given these results, an enhancement in the carbonation reaction was apparent in suspensions 2 and 3 (low pH + 5 % MgCl2 and high pH + 5 % MgCl2, respectively) as compared with suspension 1 (unaltered pH and salinity). Though the calcite peak was visible in all three suspensions, the intensity is highest in suspension 3, which could mean that the formation of carbonate mineral is more favorable in this condition. In addition, more diverse types of carbonate minerals could be formed in highly alkali suspensions due to the participation of OH− (as seen in the formation of hydromagnesite [Mg5(CO3)4(OH)2·4H2O]).
It is likely that other non-carbonate precipitation or competing reactions have occurred. The presence of non-carbonate phases strongly indicates an incomplete carbonation process. The suspensions and other experimental conditions must be further refined to achieve a maximum sequestration-efficient procedure.
Carbonate formation at different rock particle size
Particle size is also considered as an important factor affecting carbonation as it is generally accepted that the smaller the average particle size, the larger the mineral surface area per unit mass and the higher the reactivity of the particles become [15]. Results of the XRD analyses, as shown in Fig. 4, for rock samples with fine (62–125 μm) and (250–420 μm) coarse particle sizes showed that the change in the mineral composition is similar in both experimental group. In addition to the previously mentioned formation of calcite and hydromagnesite, Dolomite [CaMg(CO3)2] and Pirssonite [Na2Ca(CO3)2·2H2O] could also be formed in the carbonation reaction as indicated by the peaks that appeared at 2θ = 30.98 (d-spacing = 2.886) and 2θ = 34.98 (d-spacing = 2.565), which correspond to their respective d-spacings. The intensity of the peaks in the fine particle size set-up is slightly higher than the peaks in coarse particle size set-up. This could be an evidence that smaller particles indeed undergo faster mineral dissolution. Thus, the formation of new carbonate minerals is also slightly favorable in this condition. The coarse or fine fractions can influence additional energy input to enhance surface interactions [31]. Nonetheless, their difference is not significant and it can consequently be concluded that, within the particle sizes used in this study (62–420 μm), the effect of particle size on mineral carbonation reactions is negligible and is indicative only of the viable working range. Understanding of particle size would introduce an additional variable to be considered in identifying prospective injection points in Luzon ophiolite rocks sites.
X-ray diffraction patterns of rock samples from a Angat Ophiolites, b Zambales Ophiolite complex, and c Camarines Norte Ophiolite Complex, before (day 0) and after (60 days) subjecting to mineral carbonation experiments (1) Fine (particle size: 62–125 μm); (2) coarse (particle size: 250–420 μm). Y-axes are all on the same scale
Environmental implications of carbon mineralization
The experimental findings and methods used could be further developed to CO2 mineralization strategies at potential sites where carbon footprint could be significant, such as Bicol and Leyte, Philippines. Large geothermal power plants are in use [9] in these areas and possibly, tons of CO2 could be emitted from geothermal operations. However, mineral exploration within the vicinity of the geothermal plants is yet to be done to determine if available ophiolitic rocks are close to the site for CO2 injection. In addition, instead of emitting CO2 into the atmosphere, pipes could be connected to nearby alkaline springs for direct channeling of CO2 and consequently produce the CO2-sparged solutions. Mineral carbonation process is a relatively young technology, where many areas of this technology has yet to be explored, primarily the quantification of CO2 sequestered, as well as other parameters affecting carbonation reaction (i.e., pressure and temperature).
A future research investigation will be undertaken to assess the physical and chemical characteristics (e.g., stability) of the carbonate phases and non-carbonate phases formed after the carbonation procedure. This will give a better direction on identifying the potential commercial markets (e.g., construction materials) of these high value products.
Several research and development gaps still need to be addressed before mineral carbonation can be integrated to established power plant systems. Deployment of in situ carbonation should be given a serious chance since rock formations (ophiolites) containing high concentrations of Ca and Mg are abundant in many locations in the world.
Conclusions
A CO2 mineral sequestration procedure that utilizes alkaline spring samples was proposed. Investigations on bubbling of CO2 into alkaline springs would be beneficial to decrease the energy input in carbon sequestration studies. Bubbling of CO2 is applied to varying experimental conditions including pH, salinity and particle sizes using different sources of ophiolitic rocks. Salinity and pH were shown to significantly enhance the mineral carbonation reaction from an unaltered system (natural pH and salinity). The formation of carbonate minerals such as calcite [CaCO3], hydromagnesite [Mg5(CO3)4(OH)2·4H2O], Dolomite [CaMg(CO3)2] and Pirssonite [Na2Ca(CO3)2·2H2O] was confirmed in the XRD analysis of samples purged with CO2 at various conditions for 60 days. The observation of a newly formed carbonate-based mineral in the CO2-sparged suspensions is a good indication that the method employed is suitable for improving the interaction of CO2 and metal cations in aqueous suspensions.
The experiment with controlled particle sizes gave similar results in the x-ray diffraction analysis, hence it can be inferred that within the particle size range used in this study, 62–420 μm, the effect of particle sizes in mineral carbonation process is negligible. The results are yet to be applied at a scale sufficient to show their industrial viability and additional parameters, such as presence of organic constituents, should be considered in future studies. An interference study with other gases (e.g., of other green house gases) is also an interesting work in the future.
References
Alexander G, Maroto-Valer M, Gafarova-Aksoy P (2007) Evaluation of reaction variables in the dissolution of serpentine for mineral carbonation. Fuel 86:273–281
Arcilla CA, Pascua CS, Alexander WR (2011) Hyperalkaline groundwaters and tectonism in the Philippines: significance of natural carbon capture and sequestration. Energy Procedia 4:5093–5101
Barros N, Oliveira G, Lemos de Sousa MJ (2012) Environmental impact assessment of carbon capture and sequestration: General overview. “IAIA12 Conference Proceedings” Energy Future: The Role of Impact Assessment. In: 32nd Annual Meeting of the International Association for Impact Assessment, 27 May–1 June 2012, Centro de Congresso da Alfândega, Porto, Portugal
Big Sky Carbon, Sequestration Partnership. http://www.bigskyco2.org/research/geologic/basaltproject. Accessed 26 Feb 2015
CarbFix. http://www.or.is/en/projects/carbfix. Accessed 26 Feb 2015
Chavagnac V, Ceuleneer G, Monnin C, Lansac B, Hoareau G, Boulart C (2013) Mineralogical assemblages forming at hyperalkaline warm springs hosted on ultramafic rocks: a case study of Oman and Ligurian ophiolites. Geochem Geophys Geosyst 14:2474–2495
Cipolli F, Gambardella B, Marini L, Ottonello G, Zuccolini MV (2004) Geochemistry of high-pH waters from serpentinites of the Gruppo di Voltri (Genova, Italy) and reaction path modeling of CO2 sequestration in serpentinite aquifers. Appl Geochem 19(5):787–802
Dilmore RM, Allen DE, McCarthy Jones JR, Hedges SW, Soong Y (2008) Sequestration of dissolved CO2 in the oriskany formation. Environ Sci Technol 42:2760–2766
First Gen Corporation. http://www.firstgen.com.ph/OurAssets.php. Accessed 20 Feb 2015
Gerdemann SJ, O’Connor WK, Dahlin DC, Penner LR, Rush H (2007) Ex situ aqueous mineral carbonation. Environ Sci Technol 41:2587–2593
Ghanbari S, Al-Zaabi Y, Pickup GE, Mackay E, Gozalpour F, Todd AC (2006) Simulation of CO2 storage in saline aquifers. Chem Eng Res Des 84(9):764–775
Huijgen WJJ, Ruijg GJ, Comans RNJ, Witkamp GJ (2006) Energy consumption and net CO2 sequestration of aqueous mineral carbonation. Ind Eng Chem Res 45:9184–9194
Jonckbloedt RLC (1998) Olivine dissolution in sulphuric acid at elevated temperatures—implications for the olivine process, an alternative waste acid neutralizing process. J Geochem Exp 62:337–346
Keleman PB, Matter J (2008) In situ carbonation of peridotite for CO2 storage. Proc Natl Acad Sci 105(45):17295–17300
Kodama S, Nishimoto T, Yamamoto N, Yogo K, Yamada K (2008) Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 33:776–784
Lackner KS, Wendt CH, Butt DP, Joyce EL, Sharp DH (1995) Carbon dioxide disposal in carbonate minerals. Energy 20:1153–1170
Lal R (2008) Carbon sequestration. Philos Trans R Soc B 363:815–830. doi:10.1098/rstb.2007.2185
Liu Q, Maroto-Valer MM (2012) Studies of pH buffer systems to promote carbonate formation for CO2 sequestration in brines. Fuel Process Technol 98:6–13
Liu Q, Maroto-Valer MM (2010) Investigation of the pH effect of a typical host rock and buffer solution on CO2 sequestration in synthetic brines. Fuel Process Technol 91:1321–1329
Magbitang RA, Lamorena, RB (2013) Mineral carbonation process in ophiolite complexes during the CO2-purged aqueous solutions exposure. In: Proceedings of the Annual International Conference on Chemistry, Chemical Engineering, and Chemical Process (CCECP 2013). doi:10.5176/2301-3761_CCECP.38
Maroto-Valer MM, Fauth DJ, Kuchta ME, Zhang Y, Andresen JM (2005) Activation of magnesium rich minerals as carbonation feedstock minerals for CO2 sequestration. Fuel Process Technol 86:1627–1645
Matter JM et al (2011) The CarbFix pilot project-storing carbon dioxide in basalt. Energy Proc 4:5579–5585
MDI-MINERAL (2011) Materials data Inc. LiveMArtormore, CA USA (XRD mineralogy database)
O’Connor WK, Dahlin DC, Nilsen DN, Rush GE, Walters RP, Turner PC (2001) Carbon dioxide sequestration by direct mineral carbonation: Results from recent studies and current status. In: 1st Annual DOE Carbon Sequestration Conference, Washington, D.C., May 2001
Olajire AA (2013) A review of mineral carbonation technology in sequestration of CO2. J Petrol Sci Tech 109:364–392
Olsson J, Stripp SLS, Gislason SR (2014) Element scavenging by recently formed travertine deposits in the alkaline springs from the Oman Samail Ophiolite. Miner Mag 78(6):1479–1490
Palandri JL, Kharaka YK (2004) A compilation of rate parameters of water-mineral interaction kinetics for application to geochemical modeling. US Geological Survey, Open File Report 2004-1068
Park AA, Fan LS (2004) CO2 mineral sequestration: physically activated dissolution of serpentine and pH swing process. Chem Eng Sci 59:5241–5247
Park AA, Jadhav R, Fan LS (2003) CO2 mineral sequestration: chemically enhanced aqueous carbonation of serpentine. Can J Chem Eng 81:885–890
Sanna A, Uibu M, Caramanna G, Kuusik R, Maroto-Valer MM (2014) A review of mineral carbonation technologies to sequester CO2. Chem Soc Rev 43:8049–8080
Santos RM, Verbeeck W, Knops P, Rijnsburger K, Pontikes Y, Gerven TV (2013) Integrated mineral carbonation reactor technology for sustainable for carbon dioxide sequestration: CO2 energy reactor. Energy Proc 37:5884–5891
Shafeen A, Croiset E, Douglas PL, Chatzis I (2004) CO2 sequestration in Ontario, Canada. Part I: storage evaluation of potential reservoirs. Energy Convers Manage 45:2645–2659
Snaebjornsdottir SO, Wiese F, Fridriksson T, Armansson H, Einarsson GM, Gislason SR (2014) CO2 storage potential of basaltic rocks in Iceland and the oceanic ridges. Energy Proc 63:4585–4600
Streit E, Kelemen P, Eiler J (2012) Coexisting serpentine and quartz from carbonate-bearing serpentinized peridotite in the Samail Ophiolite, Oman. Contrib Miner Petrol 164(5):821–837
Tier S, Revitzer H, Eloneva S, Fogelholm C, Zevenhoven R (2007) Dissolution of natural serpentinite in mineral and organic acids. Int J Miner Process 83:36–46
Van Pham T, Aagaard P, Hellevang H (2012) On the potential for CO2 mineral storage in continental flood basalts-PHREEQC bach and 1D-diffusion reaction simulation. Geochem Trans 13(5):1–12
Acknowledgments
The authors would like to thank Natural Sciences Research Institute (CHE-12-2-01) and Office of the Vice-Chancellor for Research and Development (Project HJR-12-483), University of the Philippines Diliman for their financial support. We are also grateful for the technical assistance of Dr. Alyssa Peleo-Alampay of the Nanoworks Laboratory, National Institute of Geological Sciences, University of the Philippines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Natural Sciences Research Institute (CHE-12-2-01) and the Office of the Vice-Chancellor for Research and Development (Project HJR-12-483) in the University of the Philippines at Diliman.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Magbitang, R.A., Lamorena, R.B. Carbonate formation on ophiolitic rocks at different pH, salinity and particle size conditions in CO2-sparged suspensions. Int J Ind Chem 7, 359–367 (2016). https://doi.org/10.1007/s40090-016-0099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-016-0099-3