Abstract
The current communication is focused on preparation and effect of various counter-ions on micelle behavior of N-methylhexadecylamine and ethylene diaminetetraacetic acid dianhydride-based carboxylate anionic gemini surfactant (G16). Structure elucidation of G16 has confirmed by FT-IR, 1H-NMR and 13C-NMR. Further, the various surface studies of G16 in aqueous solution as well as in the presence of different counter-ions have been evaluated. The aqueous solution of G16 showed CMC i.e. 1.8 × 10−2 mmol/L whereas, tremendous reduction in CMC of G16 has been observed i.e. 6 × 10−3 mmol/L in the presence of 10−1 M sodium salicylate. In addition, the Krafft temperature of G16 was found to be <0 °C, revealing higher solubility aspect of compound.
Similar content being viewed by others

Introduction
Gemini or dimeric surfactants are twin molecules of monomeric surfactants chemically linked at the hydrophilic head group by a spacer [1–6]. Any innovation in preparation of gemini surfactants has been considered important because of their huge acceptability in the numerous industrial applications viz. skin care, the production of detergents and cleaning agents, corrosion inhibition, medicine, gene transfection, genetics science, environmental protection and emulsion polymerization [7–10].
In recent years, numerous articles have been published addressing the synthesis and surface behavior of anionic gemini surfactants [11–15]. However, there are limited numbers of articles found in the literature to describe the effect of various counter-ions on surface properties of the anionic gemini surfactant with carboxylate head group [16, 17]. In the present study, we reported carboxylate anionic gemini (G16) based on EDTA dianhydride and secondary fatty amine, viz. N-methylhexadecylamine has been prepared using green solvent. It is well reported in literature that salts significantly affect the micelle behavior of surfactants. Therefore, for better consideration of the surface properties of synthesized gemini, we further examine the micellization studies in water as well as in the presence of various inorganic (NaCl, KCl) and organic (NaBenz, NaSal, Fig. 1) salts by tensiometric method. The parameters studied include surface tension (γ cmc), CMC, efficiency in surface tension reduction (C20), maximum surface excess (Γ cmc), and the occupied area per molecule (A cmc) at the CMC. The objective of the present study is to synthesize G16 surfactant and examine the surface behavior of prepared G16 in the presence of various counter-ions. The added salts may reduce the consumption of surfactants in various formulations. In several cases, after the cleaning process surfactants are disposed in the environment; in this situation, less consumption of surfactants may also minimize the environmental problems. In addition, Krafft temperature as well as emulsification power of synthesized anionic gemini G16 has also been investigated.
Experimental
Materials
The chemicals EDTA dianhydride (98 %) was obtained from Sigma-Aldrich. N-methylhexadecylamine (97 %) was purchased from Carbosynth, India. Sodium hydroxide (NaOH), sodium chloride (NaCl, ≥98 %), potassium chloride (KCl, ≥98 %), sodium salicylate (NaSal, ≥98 %) and sodium benzoate (NaBenz, ≥98 %) were bought from S. D. Fine Chem., India. All the chemicals were used without any purification.
Methods
The functional group of synthesized compound was confirmed by Fourier transform infrared (FT-IR) spectroscopy (Perkin Elmer, UK). The infrared spectral analysis was obtained prior to neutralization of the compound and spectra confirmed the amide formation. The chemical structure of this compound was determined by proton nuclear magnetic resonance (1H-NMR) and carbon nuclear magnetic resonance (13C-NMR) with Bruker Avance-III 300 MHz. Elemental analysis and thermal stability of the surfactant were carried out with Perkin Elmer, UK.
The surface tension measurements were determined with Krüss K100 tensiometer by the platinum ring detachment technique. The platinum ring was completely cleaned and dried before every observation. The CMC and the surface tension at the CMC (γ cmc) were determined from the breakpoint of the surface tension versus the logarithm of the concentration curve. The results were accurate within ±0.1 mN/m. All measurements were carried out at 25 °C.
The conductivity of aqueous solutions of gemini surfactant was measured using automatic conductivity meter (Metzer, METZ-1001 M) having cell constant 1.01 cm−1. The conductometer was calibrated with KCl solutions prior to use [18].
The Krafft temperature was measured by the naked eye with known concentrations of aqueous solution of the synthesized gemini surfactant. This surfactant solution was taken in glass-stoppered graduated cylinders and kept in a refrigerator for 24 h, after that the cylinder was taken out and the temperature was noticed when a clear solution was obtained [19].
Emulsion stability was investigated as the time of separation of the water from the emulsion layer. Emulsion was prepared by mixing 40 ml of 0.1 wt% of the G16 aqueous solution and 40 mL of benzene at room temperature [20]. The measurement was repeated three times for high accuracy.
The tolerance of prepared anionic gemini against Ca2+ has been studied by foaming method [20, 21]. Foaming property was measured by the height of foam after shaking the solution of gemini in hard water (hardness 160 mg/L). For the evaluation of the performance of anionic gemini G16 in hard water, foaming powers of various solutions were recorded. Two different types of solutions were prepared. The first one was 1 % sodium stearate (soap) solution in hard water (1); second one was 1 % soap solution in hard water with 0.1 % of G16 (2).
Preparation and characterization of carboxylate anionic gemini surfactant
The anionic gemini surfactant was synthesized as in literature [12]. However, we herein report the modified scheme for its synthesis with higher yield that also in a short duration. G16 was prepared using EDTA dianhydride (10 mmol, 2.56 g) and N-methylhexadecylamine (20 mmol, 5.11 g) in methanol, refluxing and constantly stirred for 20 h at 50 °C. The reaction pathway is shown in Fig. 2. The solvent was removed under reduced pressure, and then residue was purified with chloroform. White powder was collected. The yield was almost 76 % (5.82 g). The characterization of G16 has been done by FT-IR, elemental analysis, 1H-NMR and 13C-NMR.
FT-IR spectra of the G16 (Nujol mulls, selected bands in cm−1): 3443.59 [N–H stretching], 2955.24 [–OH stretching for acid group], 2922.71 [–CH2 asymmetric stretching], 2853.02 [–CH3 symmetrical stretching], 1742.97 [C=O stretching of carboxylic acid], 1626.20 [C=O stretching of tertiary amide], 1462.63 [N–CH3], 1377.59 [C–N stretching], 1168.62 [–CO stretching], 909.26 [–OH deformation], 720.59 [–(CH2) n , skeletal]. Elemental analysis calculated for C44H86N4O6 (%): C, 68.88; H, 11.29; N, 7.30. Found: C, 68.09; H, 11.87; N, 7.76.
Furthermore, obtained product was neutralized with sodium hydroxide (1 M, 2 equivalents). 1H-NMR spectra of the compounds (300 MHz, CDCl3, δ in ppm): 0.821 [t, 6H, –CH3], 1.186 [m, 36H, –(CH2)13], 1.455 [m, 4H –CH2–C–N–C=O], 2.393 [m, 4H, N–CH2–CH2–N], 2.744 [s, 6H (C–O–N–CH3], 3.257–3.607 [m, 4H + 4H + 4H, CH3–N–C=O, N–C–CH2–N, N–CH2–COO]. 13C-NMR spectra of the compounds (300 MHz, CDCl3, δ in ppm): 13.95 [CH3–], 22.55 [CH3–CH2–], 26.27 [N–CH2–CH2–CH2–], 29.24 [N–CH2–CH2–CH2], 29.35–29.57 [–(CH2)10–], 31.80 [CH3–CH2–CH2–], 44.11 [CH3–N–CH2], 51.29 [N–CH2–CH2–N], 52.14 [CH3–N–CH2], 55.31, 58.82 [CH2–CO–N + CH2–CO–O], 172.24 [N–C=O], 176.46, [O–C=O]. The 1H and 13C-NMR spectra of the gemini surfactant are shown in Figs. 3 and 4.
Results and discussion
Surface properties
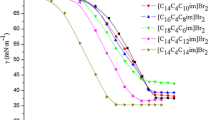
Surface properties viz. surface tension (γ cmc), CMC, efficiency in surface tension reduction (C20), maximum surface excess (Γcmc), occupied area per molecule (A cmc) and surface pressure (Πcmc) at the CMC have been estimated for G16. Surface tension (γ) of the prepared gemini in aqueous solution commutes with the concentrations of surfactants. The CMC value for gemini was examined from the break point of the surface tension versus concentration plot. The plots of surface tension of G16 in aqueous solution are shown in Fig. 5. The surface activity parameters of the prepared gemini surfactant along with corresponding carboxylate surfactants viz. carboxylate anionic gemini, sodium laurate (SL) and monomeric surfactant viz. sodium dodecylsulfate (SDS) are summarized in Table 1. As can be seen in Table 1, the CMC value of G16 was much lesser than conventional monomeric surfactant, and corresponding carboxylate surfactants. This result suggests that G16 surfactant has superior micelle forming ability at low concentration, and also much effective in reducing the surface tension as compared to corresponding analogs. The CMC values of prepared anionic gemini have further been examined by electrical conductivity measurement. The plots of specific conductance (k) against G16 concentration are presented in Fig. 6. The CMC value obtained by the conductivity measurement is in good agreement with value determined by tensiometric measurement (Table 1). The effect of inorganic salts i.e. NaCl, KCl and organic salts i.e. NaBenz, NaSal on surface activities of G16 was also examined via tensiometric measurements. Table 2 depicts the micellization parameters of G16 with inorganic and organic salts, which exhibit the increase in surface activity concomitant lower CMC with the increase of salt concentration. Data shown in Table 2 reflect that organic counter-ions show superior surface properties compared to inorganic counter-ions. It may be that organic salts demonstrate better micellization due to aromatic anionic moieties. The CMC values at various concentrations of inorganic and organic salts are exhibited in Fig. 7. On perusal of the Fig. 7, it can be noticed that the CMC values of G16 with the electrolytes indicated that it keep decreasing on increasing the concentration of electrolytes. The observed reduction in the CMC value, related with the salt addition, is because of decrease in the electrostatic repulsion between head groups in the presence of the more counter-ions from the electrolyte. The CMC values reduced effectively in the presence of various concentration of salts and followed the pattern as NaSal > NaBenz > KCl > NaCl. Among the salts used, the effects of organic salts are found to be more prominent as compared to inorganic salts. NaBenz has a carboxylate group, whereas NaSal contains a carboxylate as well as a hydroxyl group connected to the benzene ring (Fig. 1). These organic salts possess good surface activity and high water solubility, which magnify the solubility of solutes in aqueous solution, and enhance the surface activities of G16 in counter-ions observation.
The saturation adsorption values (Γcmc) at the air/water interface and the occupied area per molecule (A cmc) of gemini were calculated from the Gibbs adsorption equation [22].
Here, γ is the surface tension, C is the surfactant concentration, T is the absolute temperature, R is the gas constant (8.31 J mol−1 K−1), N A is the Avogadro’s number and n is the number of ionic species. For gemini, several researchers have used 2 or 3 for n. The values of A cmc in aqueous solution without salt, in Table 1, are based upon n = 3, whereas in Table 2, n is assumed to be 1 in the presence of various electrolytes [17, 23]. In comparison to pure G16 solutions, the G16 solutions with counter-ions have considerable preference to be adsorbed at the air/water interface. The presence of counter-ions reduces the repulsion among the head groups and more G16 molecules can be adsorbed at the interface. In the presence of counter-ions, the values of A cmc also decrease. This reduction is observed due to the progressive charge shielding and closer packing of the G16 ions at the surface.
The value of surface pressure at the CMC (Πcmc) was obtained for gemini using equation:
Here, γ 0 is the surface tension of solvent and γ cmc refers to the surface tension of surfactant solution at the CMC. This parameter demonstrates maximum reduction of surface tension, and the higher the Πcmc values, the greater is the effectiveness of the surfactants.
The pC20 value was also evaluated to measure the efficiency of gemini surfactant. The pC20 is an another parameter showing surface action of any surfactants indicating logarithm of the surfactant concentration required to reduce the surface tension of the solvent by 20 mN/m [1, 7]. The pC20 is calculated using the following equation [22].
Here, C20 stands for the concentration required to reduce the surface tension of the solvent by 20 mN/m.
Krafft temperature
The Krafft temperature (K T ) is defined as the temperature, where the concentration of the surfactants becomes equal to the CMC and surfactants form micelles [7]. The K T of surfactants can be used to determine their solubilization power. The lower the K T value of surfactant, the greater the solubility. Experimental result demonstrates that the K T of synthesized gemini G16 was below 0 °C (Table 1). This low Krafft point allows the use of this gemini in cold water. This reveals that excellent solubility of prepared gemini surfactant.
Emulsification power
Prepared anionic gemini has good emulsifying power towards benzene. Experimental result exhibited that the emulsion composed of similar quantity of benzene and water with 0.1 % G16 as emulsifier maintained its stability for 544 s. The duration for 10 mL water phase to separate from the mixture was used to evaluate the emulsification power. The longer the duration, higher is the emulsifying power.
Performance in hard water
Synthesized gemini surfactant, G16, proved the good efficiency to use in hard water. Because these are EDTA-based gemini surfactants and EDTA is well known to be dexterous metal ion trapper. Therefore, sodium salts of EDTA trap the calcium ion of hard water and enhance the capacity of these surfactants in hard water. Foaming method was applied for determining the ability of gemini in hard water with the sodium stearate (soap). Table 3 depicted that the prepared gemini had higher foam stability than sodium soap.
Thermal analysis
Thermo gravimetric analysis (TGA) has been carried for the evaluation of thermal stability of synthesized gemini surfactant. The thermogram of the dried powder of G16 is illustrated in Fig. 8. This finding demonstrates that prepared surfactant is thermally stable up to 150 °C, and the thermal disintegration starts above 180 °C. This measurement demonstrates that this surfactant has good thermal stability too.
Conclusions
In this article, we have produced carboxylate anionic gemini (G16) with 76 % yield. The yield reported is better than obtained elsewhere [12]. The newly synthesized gemini showed Krafft temperature below than 0 °C and much lower CMC than conventional monomer and corresponding carboxylate gemini too. Low CMC of G16 would be lucrative parameter of industrial applicability of compound. The data generated by interaction of G16 with organic and inorganic electrolytes show that its performance would improve in the presence of counter-ions. It further makes G16 to be a surfactant of formulation choice. In the presence of inorganic and organic salts, CMC value of G16 was obtained to be in the order: NaSal < NaBenz < KCl < NaCl. Inorganic salts are little bit slower in performance to organic salts. Organic salts decreased the CMC much better as compared to inorganic salts because the benzene ring of organic salts has hydrophobic behavior. Therefore, organic salts demonstrated more effectively from the perspective of surface activity. The surface properties of G16 with studied electrolytes indicate that mixture of salts with G16 found to be very promising for the formulation where electrolytes are essential. In addition, G16 and its combination with electrolytes may help the commercial formulations to perform better with lesser deterioration to the environment, as their CMC is too low. Therefore, they do not affect the environment adversely. On the basis of much less CMC values of anionic gemini with counter-ions, it is suggested that it may be used for commercial formulation to reduce the consumption of surfactants. As prepared surfactant G16 is an EDTA-based gemini, therefore, it can be used in various detergents formulations and may give superior performance even in hard water.
References
Menger FM, Littau CA (1991) Gemini-surfactants: synthesis and properties. J Am Chem Soc 113:1451–1452
Sohrabi B, Bazyari A, Hashemianzadeh M (2010) Effect of ethylene glycol on micellization and surface properties of gemini surfactant solutions. Colloids Surf A 364:87–93
Tsubone K, Ghosh S (2003) Micelle ionization degree of anionic gemini surfactant having N, N-dialkylamide and carboxylate groups. J Surf Deterg 6:225–229
Tsubone K, Ghosh S (2004) Micellization of an anionic gemini surfactant having N, N-dialkylamide, carboxyl, and carboxylate groups in aqueous NaCl solutions. J Surf Deterg 7:47–52
Tikariha D, Kumar B, Ghosh S, Tiwari AK, Barbero N, Quagliotto P, Ghosh KK (2013) Interaction between cationic gemini and monomeric surfactants: micellar and surface properties. J Nanofluids 2:316–324
Das S, Naskar B, Ghosh S (2014) Influence of temperature and polar organic solvents (isopropanol and 1, 4-dioxane) on the micellization of cationic gemini surfactant (14-4-14). Soft Matter 10:2863–2875
Zana R (2004) Gemini surfactants: synthesis, interfacial and solution-phase behavior, and application. Marcel Dekker, New York
Kumar N, Tyagi R (2014) Industrial applications of dimeric surfactants: a review. J Dispers Sci Technol 35:1–10
Liu JW, Xu JJ, Liu ZW, Liu XL, Che RC (2014) Hierarchical magnetic core-shell nanostructures for microwave absorption: synthesis, microstructure and property studies. Sci China Chem 57:3–12
Kumar N, Tyagi R (2013) Dimeric surfactants: promising ingredients of cosmetics and toiletries. Cosmetics 1:3–13
Yoshimura T, Esumi K (2004) Synthesis and surface properties of anionic gemini surfactants with amide groups. J Colloid Interface Sci 276:231–238
Wattebled L, Laschewsky A (2007) New anionic gemini surfactant based on EDTA accessible by convenient synthesis. Colloid Polym Sci 285:1387–1393
Altenbach HJ, Ihizane R, Jakob B, Lange K, Schneider M, Yilmaz Z, Nandi S (2010) Synthesis and characterization of novel surfactants: combination products of fatty acids, hydroxycarboxylic acids and alcohols. J Surf Deterg 13:399–407
Ghosh S, Chakraborty T (2007) Mixed micelle formation among anionic gemini surfactant (212) and its monomer (SDMA) with conventional surfactants (C12E5 and C12E8) in brine solution at pH 11. J Phys Chem B 111:8080–8088
Chakraborty T, Ghosh S (2007) Mixed micellization of an anionic gemini surfactant (GA) with conventional polyethoxylated nonionic surfactants in brine solution at pH 5 and 298 K. Colloid Polym Sci 285:1665–1673
Sakai K, Umemoto N, Matsuda W, Takamatsu Y, Matsumoto M, Sakai H, Abe M (2011) Oleic acid-based gemini surfactants with carboxylic acid headgroups. J Oleo Sci 60:411–417
Tsubone K, Ogawa T, Mimura K (2003) Surface and aqueous properties of anionic gemini surfactants having dialkyl amide, carboxyl, and carboxylate groups. J Surf Deterg 6:39–46
Bailey AE (1995) Bailey’s industrial oil and fat products, vol 5, 5th edn. Wiley, New York
Vautier-Giongo C, Bales BL (2003) Estimate of the ionization degree of ionic micelles based on Krafft temperature measurements. J Phys Chem B 107:5398–5403
Noori S, Naqvi AZ, Ansari WH, Akram M, Kabir-ud-Din (2014) Synthesis and investigation of surface active properties of counterion coupled gemini surfactants. J Surf Deterg 17:409–417
Kumar N, Tyagi R (2015) Synthesis of anionic carboxylate dimeric surfactants and their interactions with electrolytes. J Taibah Univ Sci 9:69–74
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, New York
Perez L, Pinazo A, Rosen MJ, Infante MR (1998) Surface activity properties at equilibrium of novel gemini cationic amphiphilic compounds from arginine, bis (Args). Langmuir 14:2307–2315
Cao X, Li Z, Song X, Cui X, Wei Y, Cheng F, Wang J (2009) Effects of spacers on surface activities and aggregation properties of anionic gemini surfactants. J Surf Deterg 12:165–172
Zhu YP, Masuyama A, Kirito Y, Okahara M, Rosen MJ (1992) Preparation and properties of glycerol-based double-or triple chain surfactants with two hydrophilic ionic groups. J Am Chem Soc 69:626–632
Chen M, Luo L, Hu X, Yang J (2013) Synthesis and surface tension study of the spacer chain length effect on the adsorption and micellization properties of a new kind of carboxylate gemini surfactant. J Surf Deterg 16:327–332
Acknowledgments
Financial support from Department of Science and Technology (DST), Grant SR/FT/CS-043/2010, New Delhi, India, for this project is gratefully acknowledged. N. Kumar is thankful to DST, New Delhi for the sanction of Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kumar, N., Tyagi, R. Synthesis and surface studies of anionic gemini surfactant in the different counter-ions. Int J Ind Chem 6, 59–66 (2015). https://doi.org/10.1007/s40090-015-0032-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-015-0032-1