Abstract
Renewable and non-renewable energy sources remain the two fronts for meeting global emergent energy demand. Renewable energy sources such as crude oil, in meeting energy needs, is a function of new hydrocarbon discoveries and improving the recovery of existing oil fields. However, new crude oil discoveries are made at a decreasing rate; likewise, existing fields are at a declining phase with conventional recovery techniques not being able to produce as much as two-thirds of the oil in place. In complementing existing oil recovery techniques, research into the use of nanotechnology has emerged as a potential alternative for tertiary oil recovery scheme. Despite the promising results, there has not been any reported large-scale field application of nanotechnology in the oil and gas industry except for some small-scale field trials. In this paper, a detailed review of developments on nano-enhanced oil recovery (Nano-EOR) and its attendant challenges are presented. Furthermore, key recommendations were given for future research on Nano-EOR. While the adoption of new technologies has its associated risks, the future prospects of Nano-EOR remains very high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Demand for energy has increased over the years with global industrialization and population growth [47]. Renewable energy sources such as crude oil, in meeting energy needs, is a function of new hydrocarbon discoveries and improving the recovery of existing oil fields. Unfortunately, existing oil and gas fields are faced with production in the declining phase [47]. New oil field discoveries are limited and majorly reported in complex reservoir environments, which are mostly under high pressure and high temperature (HPHT) [2, 3, 25]. The future of the global oil and gas industry would depend largely on the development of new technologies to meet growing energy demand. The need for new technologies is required for the exploitation of new hydrocarbon discoveries and improving oil recovery from existing oil fields. Consequently, new technologies are required across the petroleum value chain, i.e. exploration (frontier exploration and basin exploitation), appraisal, field development, production, reserves growth, and field reactivation or abandonment [1, 2, 15, 16]. Recent research trend has shifted in various disciplines from the “Macro-domain” to the “Micro-domain” and “Nano-domain” and probably in the coming years to “Pico-domain” and “Femto-domain”. Already there is the talk of “Picotechnology” where matter is handled at the atomic scale. Picoparticles are considered as clusters of atoms. Although considered by futurist as hypothetical, there have been reports of the synthesis and use of gold picoparticles for catalysis. “Femtotechnology”, on the other hand, deals with the handling of agitated energy levels inside atomic cores, which would result in metastable conditions with rare attributes. “Femtotechnology” for now has no practical application and it is considered hypothetical by futuristic scientist and engineer. In other words, the summary of this is that technological advancement in the world today is achieved through scaling down. Furthermore, areas such as microfluidics, nanofluidics, nanoscience, and nanotechnology have witnessed increased research due to the advancing trend of scaling down in technological innovations. Nanoparticles are engineered to have at least a dimension in the order of 100 nm. The uniqueness of nanoparticles is tied to its size-dependent properties, which are a consequence of its large surface-to-volume ratio thereby enabling surface atoms/molecules to have a significant impact on its properties [5, 9]. Furthermore, due to the small size of nanoparticles, the amount of surface atoms is large compared to the bulk atoms. This enables the properties of nanoparticles to be governed by the surface atoms and described by the physics of quantum mechanical consideration [5]. In addition, the high surface-to-volume ratio of nanoparticles ensures that considerable amount of free surface energy exists within them [5, 45, 49]. This explains the strong force of attraction that exists between nanoparticles when in suspension. The attraction between nanoparticles or to other molecules ensures that the free energy is minimized. Nanoparticles can be tailor-made to meet specific application depending on the property of interest [45]. The desired properties can include thermal, mechanical, chemical, electrical, optical, magnetic, etc. The discovery of oil reserves in challenging offshore location has made research into the thermal and mechanical properties of nanoparticles paramount [5, 45, 46]. The application of nanoparticles, for example, in the formulation of water-based drilling muds has improved their performance in reducing fluid loss when applied in shale formations as a result of their size-dependent properties [15, 17, 38, 49].
Nanotechnology and oil recovery process
Renewable energy sources such as crude oil, in meeting energy needs, is a function of new hydrocarbon discoveries and improving the recovery of existing oil fields. However, new crude oil discoveries are made at a decreasing rate; likewise, existing fields are at a declining phase with conventional recovery techniques not being able to produce as much as two-thirds of the oil in place. In complementing existing oil recovery techniques, research into the use of nanotechnology has emerged as a potential alternative for tertiary oil recovery scheme. “Nano-EOR” is a term used in describing the use of nanoparticles for Enhanced Oil Recovery (EOR) process. The mechanism by which nanoparticles improve the recovery of residual oil has been established via wettability alteration and interfacial tension reduction. The combination of these two may not be the only underlying mechanism as several other approaches have been offered for nano-EOR.
Wettability and interfacial tension alteration using nanoparticles
Residual oil saturation exists in reservoirs due to “oil entrapment” at the microscopic level arising from capillary forces and “oil bypass” at the macroscopic level during secondary recovery [5]. An approach to improving or enhancing the recovery of oil from rock formations would be the alteration of the wettability of such formations [26, 29, 35, 40]. A formation is said to be water wet if the angle of contact on the surface of the rock is below 90 degrees while it is said to be oil wet if the angle of contact on the surface of the rock is greater than 90 degrees. A water wet formation would have oil easily extracted from it than an oil-wet formation [29, 40]. The use of nanoparticles in improving oil recovery has been reported in several literatures. Son, Kim, Lee, Kim, and Sung (2014) investigated the use of nanoparticle-stabilized oil/water emulsions as EOR agents in a column of silica bead having mineral oil. The results showed an increase in the rate of oil recovery of about 11% after water flooding. This can be ascribed to larger pressure variance through the column, which enables the remaining oil in it to be produced through a piston effect. Ogolo et al. [29] carried out a comparative study on the effect of eight different nanoparticle oxides (oxides of magnesium, zinc, aluminum, silicon, tin, nickel, zirconium, and iron) on oil recovery under surface conditions. The dispersion media used in preparing the nanofluids include water, ethanol, diesel, and brine. The experimental results from the study showed that the oxides of Aluminum and Silicon would be suitable for EOR application when they are dispersed in water and brine. In the case of ethanol being the dispersion media, it was discovered that silane-treated oxide of silicon gave the most oil recovery. It was also discovered from the experiment that the oxide of Aluminium reduces the viscosity of oil while that of Silicon alters the wettability of rock as well reducing the interfacial tension between oil and water.
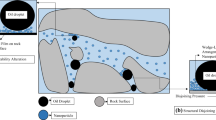
The phenomenon of wettability modification and interfacial energy decrease is widely seen as the mechanism through which nanoparticles work in enhancing the recovery of oil from oil-bearing formations [29, 31, 35, 40]. Onyekonwu and Ogolo [31] looked at the ability of polysilicon nanoparticles which are hydrophilic and lipophilic (LHPN), polysilicon nanoparticles which are hydrophobic and lipophilic (HLPN), and polysilicon nanoparticles which are neutrally wet (NWPN) on improving oil recovery through wettability alteration. The experimental results obtained from the study indicates that HLPN and NWPN improved oil recovery in water wet formations and the mechanism through with this was achieved was through wettability alteration and interfacial tension reduction when the dispersion medium for the nanoparticles is ethanol. The LHPN was observed to increase oil recovery in oil formations that are water wet. Also buttressing the phenomenon of wettability modification and interfacial energy decline of nanoparticles was the work of [36]. They evaluated and related the effectiveness of modified silica nanoparticles in improving the recovery of oil from two oil reservoirs: light Iranian and intermediate oil reservoirs. Results from their experimental study showed that in the presence of silica nanoparticles, there was a reduction in the interfacial tension in the case of light and intermediate oil. Furthermore, contact angle measurement showed that there was an alteration of wettability of rock from oil-wet condition to water-wet condition. They explained that the presence of the nanoparticles in aqueous solutions in porous media improved the recovery of oil through the reduction of interfacial energy and modification of wettability from oil wet to water wet. Trapped oil droplets in the porous media are mobilized through interfacial tension reduction (Fig. 1). Also, the role of capillary pressure was changed from being a barrier force to a driving force through wettability alteration. The effect of nanoparticle size on the incremental oil recovery through wettability alteration was captured through contact angle measurement in the work of [12, 13]. Contact angle measurements, residual oil saturation, and displacement efficiency estimation were carried out for Nano fluids prepared at 0.05 wt% for single particle sizes 7 nm, 14 nm, and 40 nm. Experimental results indicated that higher residual oil saturation (\(S_{\text{or}}\)) after waterflooding would give higher incremental oil recovery using nanofluids prepared for a given particle size (in this case, 7 nm; Fig. 2). However, a decrease in the incremental oil recovery and displacement efficiency after nano-EOR at relatively similar residual oil saturation was observed with increase in nanoparticle size. It was also observed that the contact angle measured for the aqueous phase decreases with nanoparticle size (Fig. 2). The trend observed by the authors shows that nanoparticle size improve the displacement mechanism of residual oil during nano-EOR. This is due to their ability to flow easily through pore throats thereby enhancing residual oil mobilization for incremental oil recovery. The trend observed with contact angle measurement indicates the impact of nanoparticle size on wettability alteration. Furthermore, the influence of initial rock wettability on the nano-EOR process is also important. Nano-EOR is reported to be more efficient in intermediate wet rocks with small sized nanoparticles (Fig. 3). This is due to the easy disconnection of trapped residual oil and mobilization for incremental recovery. Figure 4 shows the effect of nanoparticle concentration on the displacement efficiency of the nano-EOR process. Peak displacement efficiency was obtained at a nanoparticle concentration of 0.05 wt% after which there was a decrease in the values for the displacement efficiency. One of the associated challenges with the use of nanoparticles is the determination of the optimal amount of nanofluid for EOR operations. This has become imperative because highly concentrated nanofluids have the tendency to block pore throats in porous media leading to lower displacement efficiency (Fig. 4, > 0.05 wt%). Furthermore, the economic implications of determining an optimal concentration for the nano-EOR process would be affected by production cost of nanoparticles. Traditional synthesis approach to nanoparticles involving physical and chemical means would incur huge production costs if applied towards nano-EOR process [12, 13].
Effect of initial rock wettability on displacement efficiency and incremental oil recovery of the nano-EOR process (Concentration of nanofluids: 0.05 wt%, nanoparticle size: 7 nm and injection rate: 0.2 cm3/min). The wettability of the rock: WW (water wet), IW (intermediate wet), and OW (oil wet). [12, 13]
Although the study of nanoparticles as wettability modifiers is still relatively new with no clear-cut explanation to the mechanism of wettability alteration, it is important to point out that the concentration of nanoparticles in solution may affect the rock wettability through rock–fluid interaction. Roustaei and Barzagadeh [34] investigated the effect of nanoparticle concentration in aqueous solution on the wettability of carbonate rocks with the sole purpose of determining the optimal amount of nanofluid for addition to core samples. Their result indicates that a concentration of 4 g/dL was the optimum concentration of nanofluid that would significantly alter the wettability of the surface of the rock from oil wet to water wet. A rock surface is either water wet or oil wet based on the surface energy and interfacial energy [40]. The spreading coefficient of water, S, on the surface of a solid that is oil wet and in communication with water and oil can be related to the interfacial tension between each phase by the following expression:
where \(S_{\text{W}}\) is the spreading coefficient of water on the solid surface, \(\gamma_{\text{O/S}}\) is the interfacial energy between oil and solid, \(\gamma_{\text{W/S}}\) is the interfacial tension between water and solid, and \(\gamma_{\text{O/W}}\) is the interfacial tension between oil and water. In Eq. (1) when the interfacial tension between oil and water is reduced, the spreading coefficient of water increase thereby making the surface more water wet. In addition, if the interfacial tensions \(\gamma_{\text{W/S}}\) and \(\gamma_{\text{O/W}}\) are both reduced, the rock also becomes more water wet. The spreading of water and contact angle reduction is due to a force balance between adhesion and cohesion. Force of adhesion exists between the molecules of water and the rock surface while the force of cohesion exists between the molecules of water. When interfacial tension between oil and water, water and solid is reduced, the force of adhesion between water molecules and the rock surface becomes more pronounced thereby increasing the spreading coefficient of water and making the rock more water wet. The spreading coefficient of oil, \(S_{\text{o}}\), on the surface of a solid which is water wet and in communication with water and oil can also be related to the interfacial tension between each phase by the following expression:
where \(S_{\text{O}}\) is the spreading coefficient of oil on the solid surface, \(\gamma_{\text{O/S}}\) is the interfacial energy amongst oil and solid, \(\gamma_{\text{W/S}}\) stands for the interfacial tension amongst water and solid, and \(\gamma_{\text{O/W}}\) denotes the interfacial energy among oil and water. In Eq. (2) when the interfacial tension between oil and water is reduced, the spreading coefficient of oil increases thereby making the surface more oil wet. In addition, if the interfacial tensions \(\gamma_{\text{O/S}}\) and \(\gamma_{\text{O/W}}\) are both reduced, the rock also become more oil wet. The spreading of oil and contact angle increment is due to a force balance between adhesion and cohesion. Force of adhesion exists between the molecules of oil and the rock surface while the force of cohesion exists between the molecules of oil. When interfacial tension between oil and water, oil, and solid is reduced, the force of adhesion between oil molecules and the rock surface becomes more pronounced, thereby increasing the spreading coefficient of oil and making the rock more oil wet.
However, the mechanism of wettability alteration for nanofluids is still in its infancy and many kinds of literature have reported different approach [34, 40], but wettability alteration for nanoparticles is always from oil wet to water wet. Another literature reports on wettability modification involving the use of surfactants and nanofluid mixtures [40]. Furthermore, such study did not provide conclusive evidence on the impact of nanoparticles on wettability alteration, as surfactants are already known wettability modifiers. Fluid–rock interactions and fluid–fluid interactions may also explain how nanofluid affects wettability alteration. Sandstone formations are mainly made up of minerals of silica whose charge is negative at neutral pH while dolomite is mainly positively charged due to its large content of calcium and magnesium ions [48]. When nanofluid made up of positively charged nanoparticles flows in a porous sandstone media, which is oil wet, some of the nanoparticles may adsorb on the rock surface while others may adhere to the oil–water interface. A plausible explanation for the fluid–rock interaction could be that when nanoparticles in the nanofluid comes in contact with the charged sandstone rock surface, charge interaction between the nanoparticle and rock allows for the nanoparticles to be adhered to the rock surface. This disrupts whatever molecular attachment amongst the rock surface and the oil molecules accountable for the oil-wet condition. A rock surface initially water wet could become oil wet if it is exposed to oil for some time. During this exposure period, it can be deduced that a dipole–dipole attraction could have resulted between the rock surface and the oil molecules allowing for oil-wet conditions. The charged rock surface can temporarily cause an uneven charge distribution in a non-polar oil molecule allowing temporary charges to build upon the oil molecules. For example, a positively charged carbonate rock if exposed to oil, which is non-polar for a certain period, can attain dipoles due to the attraction of electrons in the oil molecules towards the positively charged carbonate rock. This allows oil-wet condition to be attained with the carbonate rock. The same phenomenon can be attributed to nanofluids containing nanoparticles when they interact with the charged surface. The charged rock surface can temporarily cause an uneven charge distribution in the nanofluid allowing temporary charges to build up. The charged nanoparticles on the rock surface can form hydrogen bonds with water molecules thereby attracting water molecules on the rock surface. This reduces the interfacial tension between rock surface and water, \(\gamma_{\text{W/S}}\) and allows water-wet condition to be created on the rock surface. At the water–oil interface, the nanoparticles form hydrogen bond with the water molecules thereby increasing the interfacial tension between the oil and water, \(\gamma_{\text{O/W}}\). This increase in interfacial tension between oil and water allows for greater capillary pressure, which will ensure a greater imbibition of water into lesser pores of the rock and hence improved oil recovery [34].
Disjoining pressure due to nanoparticles
It is observed that when a solution of nanofluid encounters an oil droplet on a solid surface, two contact lines arise [40]. These lines are separated by a layer of well-ordered nanoparticle structures, which brings about high disjoining pressures thereby causing the wedge-like spreading of the nanoparticles before the inner line and after the outer line (Fig. 1). The generation of this well ordered wedge-like structure of nanoparticles is driven by the injection pressure of the nanofluids thereby forcing the nanoparticles into a confined region [42]. This arrangement increases the entropy of the nanofluids due to the greater freedom of the NPs in the nanofluids thereby resulting in the exertion of a disjoining pressure. The identified forces behind the structural disjoining pressure include van der Waals, Brownian motion, and electrostatic repulsion. The electrostatic repulsion between the nanoparticles will be higher due to their small size thereby giving a larger structural disjoining pressure. The disjoining pressure arises due to the ability of nanoparticle dispersions to interact with the rock surface, thereby causing an imbalance in the interfacial forces between the oil and the solid phase. The magnitude of the disjoining force arising from the nanoparticle dispersions depends on nanoparticle parameters (size, volume fraction, and stability), rock properties, salinity, and temperature. The presence of salts and electrolytes would reduce the repulsive force between nanoparticles leading to aggregation or agglomeration. Nanoparticle aggregates would reduce the structural disjoining pressure as ordering of particles inside the wedge structure would not occur due to a reduction in the overall entropy of the system. Nanoparticles in the dispersed medium achieve lesser freedom at under this state of aggregation.
Propagation of nanoparticles in porous media
Most oilfield environment are under conditions of high temeperature, salinity, and pressure, while rock properties are not uniformly distributed [26, 40, 48]. The understanding of the propagation and adsorption of nanoparticles is crucial in their use in such environment. The transportation of nanoparticles in porous media takes place through a combination of diffusion, convection, and hydrodynamic forces. ShamsiJazeyi et al. [40] cited three mechanisms, which affect the transmission of nanoparticles in a medium that is porous, and this includes filtration through a physical process, chemical stability in solution, adsorption or retention on the surface of a medium which is porous and log jamming. Physical filtration is connected with particle size being larger than pore size in porous media and it is independent of nanoparticle dispersibility [40], depending on the shape, size distribution, and aspect ratio of nanoparticles. Chemical instability of nano-dispersions often leads to nanoparticle aggregation. This occurs because of hydrophobic and Van der Waals interactions that can be inhibited through polymer coatings on it. The chemical solution stability of nanoparticles is affected by salinity and presence of divalent ions. Miranda et al. [26] studied the stability and mobility of functionalized silica nanoparticles at high salt concentration and temperature for enhanced oil recovery purposes. They discovered that the presence of ions could modify the transport behavior of nanoparticles. This was observed through an increase in the coefficients of diffusion for nanoparticles with increasing amount of salt. In addition, nanoparticle adsorption on the exterior of rocks and the presence of salt solutions tend to impact on interfacial tension through modification of electrostatic potential and hydration characteristics of the salt ions near the nanoparticle surface [26].
Yu, et al. [48] investigated the essential carriage and retaining properties of nanoparticles under extreme salinity by means of real core supplies. The stability of the nanoparticles was found to be reliant on the amount of communication amongst nanoparticles, a solution containing salt ions and exterior of the porous medium, which affects retention and retardation. The presence of divalent ions may affect the propagation of nanoparticles through the formation of “salt-bridge” between the ions and the nanoparticle [29]. Retention of nanoparticles on the exterior of rocks is a result of charge communication amongst the nanoparticles and the exterior of the rock. Carbon nanoparticles, which are essentially negatively charge, were not retained on sandstone mineral during a transport study of carbon nanoparticles [29]. This may be due to electrostatic force of repulsion amongst the sandstone minerals, which are charged negatively, and the negatively stimulated carbon nanoparticles.
Nanoparticles, which have polymers covalently attached to their surface, are known as Polymer-Coated Nanoparticles (PNPs). PNPs are nanoparticles whose surface has been functionalized or modified with polymer materials [41]. While some polymeric coatings can stabilize nanoparticles, they cannot prevent their adsorption on rock surfaces. The strength of nanoparticles with coatings of polyelectrolyte is also affected by the increase in salinity and pH. The use of elongated polymer chains like starch, polyacrylamide or cellulose as covering on a nanoparticle surface to make them stable in solution has not been successful at high salinities in most reservoirs because of poor solubility of the polymers. PNPs have been shown to behave in a different way under static and dynamic conditions. ShamsiJazeyi et al. [40] also mentioned the behavior of solutions of iron nanoparticles coated with poly (acrylic acid) and its transport along a porous media. It was discovered that nanoparticle size depends on the number of nanoparticles under dynamic conditions and not static. It was concluded that the forces acting on nanoparticles under dynamic conditions are absent under static conditions. This reflects how complex the effects of flow rate, permeability, etc. on nanoparticles under dynamic conditions and using static conditions to validate the chemical stability of nanoparticles may not be sufficient. The propagation of well-stabilized functionalized nanoparticles in porous media may be hindered and retarded by adsorption on the rock surface [40, 48]. PNPs, which have a charged surface, which is the same as the rock surface or less hydrophobic, may show less adsorption. This can be challenging given the salinities and divalent ion concentration present in most oil reservoirs. Table 2 shows a summary of some experimental works done on Nano-EOR.
Mechanical entrapment and log jamming
The phenomenon of mechanical entrapment by nanoparticles occurs when the size of the particles is larger than the pore size or pore throat of the reservoir formation (Fig. 5). Log jamming by nanoparticles occurs due to the accumulation of nanoparticles at the pore throat larger than the size of the nanoparticles. The accumulation of the nanoparticles at the pore throat creates additional pressure in the adjacent pore throat thereby forcing out trapped oil droplets in the pores [42]. Nanoparticle accumulation takes place when nanoparticle velocities are less than that of the carrying fluid during flow from the pore to the throat. The constriction in the flow area between the pore and the throat creates increase the velocity of the nanoparticle dispersion with water flowing faster than the nanoparticles [42]. This allows for nanoparticle accumulation in the pore throat.
Controlling the viscosity of injected fluids
The viscosity of injected fluids for oil recovery is affected by the concentration of the nanoparticles employed [40]. The increase in the viscosity of the injected fluid due to nanoparticle can be explained in terms of a reduction in the mobility of adjacent fluid molecules around the nanoparticles. The size of the nanoparticles also affects the shear viscosity of the injected fluids. Increase in the size of the nanoparticles leads to an increase in the viscosity of the injected fluids (Shah, 2009). An important parameter for monitoring the mobility of the injected fluid is the “Mobility Factor”. The mobility factor, M, is defined as the ratio of the mobility of the displacing fluid to that of the displaced fluid. This is represented mathematically in Eq. (3):
\(\lambda_{\text{i}}\) and \(\lambda_{\text{o}}\) are the mobilities of the injected fluid and oil, respectively. When the mobility ratio \(M \le 1\), the mobility of the injected fluid is less than or equal to the mobility of the displaced oil (\(\lambda_{\text{i}} \le \lambda_{\text{o}} )\). This occurs with an increase in the concentration or size of the nanoparticle in the injected fluids. The consequence of this is an even displacement front and a reduction in the incidence of viscous fingering and early water breakthrough (Fig. 6). When the mobility ratio \(M > 1\), the mobility of the injected fluid is greater than the mobility of the displaced oil (\(\lambda_{\text{i}} > \lambda_{\text{o}} )\). This occurs with a decrease in the concentration or size of the nanoparticle in the injected fluids.
Asphaltene inhibition of nanoparticles
Some conventional EOR techniques involve mixing of injected chemicals with oil which may bring about asphaltene precipitation. Under different reservior conditions such as a change in pressure and temperature, asphaltene precipitation may take place [42]. Davidson et al. [6] investigated a novel approach to inhibiting the formation of wax on the solid surface through magnetically induced heating using superparamagnetic nanoparticles. The process of magnetic induced heating was described as “Neel Relaxation”. The process used was similar to what was used in biomedicine heating and burning cancerous tissues. Experiments were carried out using superparamagnetic nanoparticles (10 nm) dispersed in water and hydrocarbon fluid. For solids, the nanoparticles were embedded in a solid film called “nanopoint”. For a static fluid, the heat was generated in a linear proportion with the concentration of nanoparticles. It was also discovered that heat transfer from the nanopoint to the flowing fluid was three times greater compared to the static fluid. The suspension containing the superparamagnetic nanoparticles can be injected around the wellbore region and oil transportation pipelines. The application of the magnetically induced heating would reduce wax deposition or hydrate formation. Precipitation of asphaltene from crude oil can negatively affect crude oil production and transportation. This is can occur due to changes in pressure, temperature, and composition of the crude oil. Changes in composition often occur because of mixing of oil with injected solvent during EOR operations. It is, therefore, imperative to find an inhibitor, which can either prevent or delay the precipitation of asphaltene from crude oil. Mohammadi et al. [27] investigated the effect of TiO2, ZrO2, and SiO2 fine nanoparticles potentially stabilizing asphaltene particles in the oil. The authors’ titrated n-Heptane with dead oil samples obtained from Iranian oil reservoirs in the presence of the nanofluids. Polarized light microscopy was applied to determine the onset of asphaltene precipitation. It was discovered that TiO2 nanoparticles could only effectively prevent asphaltene precipitation under acidic conditions compared to basic conditions. Using FTIR spectroscopy, TiO2 nanoparticles prevented the precipitation of asphaltene through the formation of hydrogen bonds under acidic conditions. The other nanoparticles, ZrO2, and SiO2 were also observed to stabilize asphaltene nanoaggregates under acidic conditions through the formation of hydrogen bonds. Under basic conditions, the ZrO2 and SiO2 nanoparticles were unable to prevent the precipitation of asphaltene due to the inability to form hydrogen bonds. Nanoparticles prevent asphaltene precipitation through adsorption of the asphaltene molecules on its surface (Fig. 7). Asphaltene adsorption on nanoparticle surface has been reported to be affected by asphaltene content, temperature, and contact time [42]. A large contact time allows for more asphaltene adsorption on the nanoparticle surface. Depending on the type of nanoparticle employed, asphaltene adsorption can be achieved within a short contact time.
Challenges for nano-EOR
Various challenges still exist and this has prevented the full application of nanotechnology in the oil and gas industry.
Nanoparticle synthesis/production
A key challenge, which has been identified among others towards the use of nanotechnology in the petroleum industry, relates to the production of nanomaterials. Large-scale production of nanomaterials for use in the industry is expensive going by conventional methods of synthesis. The expensive nature of nanoparticles can be related to the non-standardized approach to its production [5]. An inexpensive and cheap procedure for nanoparticle production is vital for its field application in the oil and gas industry. As technology advances in the coming years, new and efficient approach would evolve towards nanoparticle production. The use of materials and resources domiciled in countries in providing solutions to challenges encountered in the petroleum industry has been encouraged by laws such as the local content act. This has stimulated increased research into potential application of agricultural waste in nanoparticle synthesis. The conventional physio-chemical procedures for large-scale nanoparticle production may be environmentally and economically undesirable [19, 23]. Therefore, the application of Green Nanotechnology in the petroleum industry could provide the cheap platform for nanomaterial synthesis and applications. The green synthesis of nanoparticles is dependent on a couple of factors which include: the nature of plant extract, the pH of the reaction medium, reaction temperature, electrochemical potential of metal ions in solutions, concentration of metal ions, and the reaction time (Makarov et al. 2014). Makarov et al. (2014), described the mechanism describing the biological synthesis of metal nanoparticles as comprising of three major steps: activation phase, growth phase, and termination phase. In the activation phase, the metal ions in solution are reduced to metal atoms by the biologically active molecules in the plant extract after which the atom nucleation commence. In the growth phase, nanoparticles tend to coalesce into larger particles and this trend is often accompanied by increasing thermodynamic stability of the nanoparticles formed. The process by which nanoparticles coalesce into larger ones is known as “Ostwald Ripening”. The termination phase is where the morphology of the nanoparticle is determined. This is largely dependent on the ability of the bio-molecules to stabilize the nanoparticles into the most energetically favorable conformation.
Stability of nano-suspensions
Besides this, other factors militate against the full application of nanomaterials. Among them is the chemical stability of nanofluids under harsh reservoir conditions. This affects the propagation of these nanoparticles in porous media. These factors are important when considering the application of nanoparticles for enhanced oil recovery (EOR). Although research done so far in the area of “Nano-EOR” has shown that the field application of it might not be too far away but these few problems must be addressed for that to realized [30]. The dispersibility of nanoparticles in a continuous medium is also paramount to drilling mud performance. For example, well-dispersed nanoparticles tend to effectively and efficiently carry out pore plugging capabilities thereby ensuring wellbore and shale stability. When nanoparticles are not well dispersed, they tend to aggregate thereby reducing their efficiency as an additive. Various equipments such as sonicator, homogenizer, and high shear mixers have been reportedly used in ensuring the dispersion of nanoparticles in various continuous medium. After such high shearing with the equipment, the nanoparticle tends to aggregate after a given period due to the strong Van der Waals force of attraction between them. Steric hindrance or repulsive forces are needed to overcome the force of attraction between the nanoparticles [46]. The use of surfactants has been reported for use in stabilizing and dispersing nanoparticles in various continuous medium [39].
Predictive modeling of nano-EOR process
Another key challenge involves attaining predictable properties for nano-EOR process. Besides permeability/porosity impairment, a correlative model that relates oil recovery to different oil, rock, and nanoparticle properties would be hugely significant for potential application of nano-EOR. In addition, it would also be important for computational modeling, economical and cost effective planning of enhanced oil recovery operations involving nanoparticles.
Aversion to new technologies
Getting oil companies to embrace new technologies such as nanotechnology is becoming challenging. Most companies are risk-averse in the event of a crash in the price of crude oil in the global market. The recent crash of crude oil price in 2014 had far-reaching consequences on the output of many oil and gas operators. This has made new projects more costly, economically and technically challenging as the availability of sufficient funds for research and development becomes less available. Moreover, most operators may contemplate the use of new technology a riskier proposal than the established technologies in use. The gradual increase in the price of crude oil after the crash of 2014 may change the risk averseness of oil and gas operators towards the adoption of new technologies such as nanotechnology [5].
Key findings and recommendations for future research
A summary of various literature on laboratory studies on Nano-EOR shows that the use of Silica Nanoparticles is evident among researchers (Tables 1, 2). The choice of silica nanoparticles is based on its cheap accessibility. Large deposits of silica-enriched sand (silica content > 85%) are present along sandstone formations and shores of rivers and are among the most abundant minerals on the planet. This makes the production of silica nanoparticles viable using physical processes such as grinding after prior treatment to remove unwanted associated materials. Apart from its cheap accessibility, silica nanoparticles can undergo modification to either Hydrophobic/Lipophilic or Hydrophilic/Lipophobic through a silanization process [31]. This makes properties (chemical, rheological, thermal, and physical) of silica nanoparticles easily controlled by surface modification and its stability against gravity settling improved [26]. Aluminium oxide nanoparticles have good mobility control during oil recovery like that of nickel oxide. Both nanoparticles were reported to decrease the oil viscosity and increase the brine viscosity, respectively, thereby ensuring a good displacement front [29, 31]. Magnesium oxide nanoparticles reduce oil recovery through permeability impairment. The same phenomenon applies to the use of zinc oxide nanoparticles for oil recovery [29]. Zirconium Oxide, Silicon Oxide, and Tin Oxide nanoparticles can effectively prevent asphaltene precipitation during oil recovery [27]. A combination of these nanoparticles can be employed during oil recovery for maximum efficiency. Based on the findings of this work, the following are recommended for future research:
-
a.
Investigation of nanoparticle retention on rock surfaces during core flooding [44]. This would require the determination of nanoparticle concentration before injection and the concentration of nanoparticles in the ejected effluent fluid. Furthermore, the retention of nanoparticles and its effect on rock properties (permeability and porosity) should be investigated for different rock types.
-
b.
Investigation into the use of “Nanofluid Slugs” for residual oil mobilization should be carried out [44]. Nanofluid slugs can be obtained through alternating brine and nanofluid injection into oil-saturated core sample. The brine could help reduce the effect of nanoparticle adsorption on rock surface, as the presence of salts will desorb retained nanoparticles on the rock surface. This process may provide a possible solution porosity/permeability impairment attributed to the use of nanoparticles. Another possible advantage of this lies in the reduction of nanoparticles employed compared to a continuous injection of nanofluids.
-
c.
There is also need to investigate the relationship between nanoparticle size/morphology and recovery rate for different injection rates. The propagation of nanoparticles in porous media is function of its size variation. The morphology or shape of nanoparticles is also key in its propagation. A good understanding of the relationship between nanoparticle size/morphology and recovery rate for different injection rates would help in proper computation of pumping requirements for nano-EOR process.
-
d.
A combination of low salinity waterflooding with nanoparticles can also be investigated for different concentration of salt. The impact of varied salt concentration on the ability of the nanoparticles in reducing interfacial tension and wettability alteration would be significant.
-
e.
Predictive models for rock permeability/porosity impairment by nanoparticles are important for computational modelling, economical and cost effective planning of enhanced oil recovery operations involving nanoparticles. Besides permeability/porosity impairment, a correlative model that relates oil recovery to different oil, rock, and nanoparticle properties would be hugely significant for the potential application of nano-EOR.
Conclusion
A paradigm shift to Nano-EOR represents the future of tertiary oil recovery scheme in meeting emergent energy demand from non-renewable crude oil sources. The large number of research carried out over the past decade and more is a glowing testament to the potential inherent in nanotechnology for EOR application. Silica nanoparticles remain the most widely experimented for potential EOR applications compared to other nanoparticles. Moreover, all experimented nanoparticles have the potential to impact on oil recovery with varied results. The future prospect on the emergence of the Nano-EOR scheme would depend on further research in the following areas identified in the recommendation section of this article. The adoption of new technologies has its associated risks but the potential for nano-EOR remains very high.
References
Abdo, J., Danish Haneef, M.: Nano-enhanced drilling fluids: pioneering approach to overcome uncompromising drilling problems. J. Energy Res. Technol. 134, 1–6 (2012)
Afolabi, R.O., Orodu, O.D., Efeovbokhan, V.E.: Properties and application of Nigerian bentonite clay deposits for drilling mud formulation: recent advances and future prospects. Appl. Clay Sci. 143, 39–49 (2017)
Afolabi, R.O., Orodu, O.D., Efeovbokhan, V.E., Rotimi, O.J.: Optimizing the rheological properties of silica nano-modified bentonite mud using overlaid contour plot and estimation of maximum or upper shear stress limit. Cogent. Eng. 4, 1–18 (2017)
Al-Anssari, S., Barifcani, A., Wang, S., Maxim, L., Iglauer, S.: Wettability alteration of oil-wet carbonate by silica nanofluid. J. Colloid Interface Sci. 461, 435–442 (2016)
Bennetzen, M.V., Mogensen, K.: Novel applications of nanoparticles for future enhanced oil recovery. International Petroleum Technology Conference, pp. 1–14. Society of Petroleum Engineers, Kuala Lumpur (2014)
Davidson, A., Huh, C., Lawrence, S.: Focused magnetic heating utilizing superparamagnetic nanoparticles for improved oil production. SPE International Oilfield Nanotechnology Conference and Exhibition, pp. 1–16. Society of Petroleum Engineers, Noordwijk (2012)
El-Diasty, A.I.: The potential of nanoparticles to improve oil recovery in bahariya formation, Egypt: an experimental study. SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia (2015)
Ehtesabi, H., Ahadian, M.M., Taghikhani, V.: Enhanced heavy oil recovery using TiO2 nanoparticles: investigation of deposition during transport in core plug. Energy Fuels 29, 1–8 (2012)
Gerogiorgis, D.I., Reilly, S., Vryzas, Z., Kelessidis, V.C.: Experimentally validated first-principles multivariate modeling for rheological study and design of complex drilling nanofluid systems. SPE/IADC drilling conference and exhibition, pp. 1–11. Society of Petroleum Engineers, Hague (2017)
Giraldo, J., Benjumea, P., Lopera, S., Cortes, F.B., Ruiz, M.A.: Wettability alteration of sandstone cores by alumina-based nanofluids. Energy Fuels 27, 3659–3665 (2013)
Haroun, M.R., Alhassan, S., Ansari, A.A., Kindy, N.A.M., Sayed, N.A., Kareem, B.A.A., Sarma, H.K.: Smart nano-EOR process for abu dhabi carbonate reservoirs. Abu Dhabi international petroleum conference and exhibition, Abu Dhabi, UAE (2012)
Hendraningrat, L., Li, S., Torsater, O.: Effect of some parameters influencing enhanced oil recovery process using silica nanoparticles: an experimental investigation. SPE Reservoir Characterization and Simulation Conference and Exhibition, Abu Dhabi, UAE (2013)
Hendraningrat, L., Li, S., Torsaeter, O.A.: Coreflood investigation of nanofluid enhanced oil recovery. J Pet Sci Eng 111, 128–138 (2013)
Hendraningrat, L., Torsæter, O.: Understanding fluid-fluid and fluid-rock interactions in the presence of hydrophilic nanoparticles at various conditions. SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia (2014)
Hoelscher, K.P., Stefano, G., Riley, M., Young, S.: Application of nanotechnology in drilling fluids. SPE International Oilfield Nanotechnology Conference (pp 1–7). Society of Petroleum Engineers, Noordwijk (2012)
Ismail, A.R., Aftab, A., Ibupoto, Z.H., Zolkifile, N.: The novel approach for the enhancement of rheological properties of water based drilling fluids using multi-walled carbon nanotubes, nanosilica and glass beads. J. Petrol. Sci. Eng. 139, 264–275 (2016)
Jung, C.M., Zhang, R., Chenevert, M., Sharma, M.: High performance water-based mud using nanoparticles for shale reservoirs. Unconventional Resources Technology Conference, pp. 1–7. Society of Petroleum Engineers, Denver (2013)
Karimi, A., Fakhroueian, Z., Bahramian, A., Khiabani, N.P., Darabad, J.B., Aniz, R., Arya, S.: Wettability alteration in carbonates using zirconium oxide nanofluids: EOR implications. Energy Fuels 26, 1028–1036 (2012)
Kavitha, K.S., Baker, S., Rakshith, D., Kavitha, H.U., Yashwantha Rao, H.C., Harini, B.P., Satish, S.: Plants as green source towards synthesis of nanoparticles. Int Res J Biol Sci 2(6), 66–76 (2013)
Kazemzadeh, Y., Eshraghi, S.E., Kazemi, K., Sourani, S., Mehrabi, M., Ahmadi, Y.: Behaviour of asphaltene adsorption onto the metal oxide nanoparticle surface and its effect on heavy oil recovery. Ind. Eng. Chem. Res. 54, 233–239 (2015)
Li, S., Hendraningrat, L., Torsaeter, O.: Improved oil recovery by hydrophilic silica nanoparticles suspension: 2 phase flow experimental studies. In Proceedings of the IPTC 2013: international petroleum technology conference; European Association of Geoscientists & Engineers, Beijing, China (2013)
Li, S., Genys, M., Wang, K., Trosaeter, O.: Experimental study of wettability alteration during nanofluid enhanced oil recovery process and its effect on oil recovery. In: Proceedings of the SPE reservoir characterisation and simulation conference and exhibition, Abu Dhabi, UAE (2015)
Logeswari, P., Silambarasan, S., Abraham, J.: Ecofreindly synthesis of silver nanoparticles from commercially available plant powder and their anti-bacterial properties. Scientia Iranica F 20(3), 1049–1054 (2013)
Maghzi, A., Mohammadi, S., Ghazanfari, M.H., Kharrat, R., Masihi, M.: Monitoring wettability alteration by silica nanoparticles during water flooding to heavy oils in five-spot systems: a pore-level investigation. Exp. Thermal Fluid Sci. 40, 168–176 (2012)
Mahmoud, O., Nasr El-Din, H., Vryzas, Z., Kelessidis, V.: Nanoparticle based drilling fluids for minimizing formation damage in HP/HT applications. SPE international conference and exhibition on formation damage and control, pp. 1–26. Society of Petroleum Engineers, Louisiana (2016)
Miranda, C.R., De Lara, L.S., Tonetto, B.C.: Stability and mobility of functionalized silica nanoparticles for enhanced oil recovery applications. SPE International oilfield nanotechnology conference and exhibition, pp. 1–11. Society of Petroleum Engineers, Noordwijk (2012)
Mohammadi, M., Akbari, M., Fakhroueian, Z., Bahramian, A., Azin, R., Arya, S.: Inhibition of asphaltene precipitation by TiO2, SiO2, and ZrO2 nanofluids. Energy Fuels 25(7), 3150–3156 (2011)
Mohebbifar, M., Ghazanfari, M.H., Vossoughi, M.: Experimental investigation of nano-biomaterial applications for heavy oil recovery in shaly porous models: a pore-level study. J. Energy Res. Technol. 137, 1–7 (2015)
Ogolo, N.A., Olafuyi, O.A., Onyekonwu, M.O.: Enhanced oil recovery using nanoparticles. SPE Saudi Arabia section technical symposium and exhibition, pp. 1–9. Society of Petroleum Engineers, Al-Khobar (2012)
Olatunde, A.O., Usman, M.A., Olafadehan, O.A., Adeosun, T.A., Ufot, O.E.: Improvement of rheological properties of drilling fluids using locally based materials. Pet. Coal 54(1), 65–75 (2012)
Onyekonwu, M.O., Ogolo, N.A.: Investigating the use of nanoparticles in enhancing oil recovery. Nigeria annual international conference and exhibition, pp. 1–14. Society of Petroleum Engineers, Calabar (2010)
Ragab, A.M., Hannora, A.E.: A comparative investigation of nanoparticle effects for improved oil recovery—experimental work. SPE kuwait oil and gas show and conference, Mishref, Kuwait (2015a)
Ragab, A.M.S.; Hannora, A.E.: An experimental investigation of silica nanoparticles for enhanced oil recovery applications. SPE North Africa technical conference and exhibition, Cairo, Egypt (2015b)
Roustaei, A., Barzagadeh, H.: Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J Petrol Explor Prod Technol 5(1), 27–33 (2014). https://doi.org/10.1007/s13202-014-0120-3
Roustaei, A., Moghadasi, J., Bagherzadeh, H., Shahrabadi, A.: An experimental investigation of polysilicon nanoparticles’ recovery efficiencies through changes in interfacial tension and wettability alteration. SPE International Oilfield Nanotechnology Conference and Exhibition, pp. 1–7. Society of Petroleum Engineers, Noordwijk (2012). https://doi.org/10.2118/156976-MS
Roustaei, A., Saffarzadeh, S., Mohammadi, M.: An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt J. Petrol. 22(3), 427–433 (2013). https://doi.org/10.1016/j.ejpe.2013.06.010
Roustaei, A., Bagherzadeh, H.: Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J. Petrol. Explor. Prod. Technol. 5, 27–33 (2015)
Sadeghalvaad, M., Sabbaghi, S.: The effect of the TiO2/polyacrylamide nanocomposite on water-based drilling fluid properties. Powder Technol. 272, 113–119 (2015)
Salih, A.H., Elshehabi, T.A., Bilgesu, H.I.: Impact of nanomaterials on the rheological and filtration properties of water-based drilling fluids. SPE Eastern Regional Meeting. Society of Petroleum Engineers, Canton (2016)
ShamsiJazeyi, H., Miller, C.A., Wong, M.S., Tour, J.M., Verduzco, R.: Polymer-coated nanoparticles for enhanced oil recovery. J. Appl. Polym. Sci. 131(15), 1–13 (2014). https://doi.org/10.1002/app.40576
Subbiah, R., Veerapandian, M., Yun, K.S.: Nanoparticles: functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 17(35), 4559–4577 (2010). https://doi.org/10.2174/092986710794183024
Sun, X., Zhang, Y., Chen, G., Gai, Z.: Application of nanoparticles in enhanced oil recovery: a critical review of recent progress. Energies 10(345), 1–33 (2017)
Tarek, M., El-Banbi, A.H.: Comprehensive investigation of effects of nano-fluid mixtures to enhanced oil recovery. SPE North Africa technical conference and exhibition, Cairo, Egypt (2015)
Torsater, O.; Engeset, B.; Hendraningrat, L.; Suwanro, S.: Improved oil recovery by nanofluids flooding: an experimental study. SPE Kuwait international petroleum conference and exhibition, Kuwait City (2012)
Vryzas, Z., Kelessidis, V.C.: Nano-based drilling fluids: a review. Energies 10, 1–34 (2017)
Wen, D., Lin, G., Vafaei, S., Zhang, K.: Review of nanofluids for heat transfer applications. Particuology 7, 41–150 (2009)
William, J.M., Ponmani, S., Samuel, R., Nagarajan, R., Sangwai, J.S.: Effect of CuO and ZnO nanofluids in xanthan gum on the thermal, electrical and High pressure rheology of water based drilling fluids. J. Petrol. Sci. Eng. 117, 15–27 (2014)
Yu, J., Berlin, J.M., Lu, W., Zhang, L., Kan, A.T., Zhang, P., Tomson, M.B.: Transport study of nanoparticles for oil field application. SPE international conference on oilfield scale, pp. 1–16. Society of Petroleum Engineers, Aberdeen (2010)
Zakaria, M.F., Husein, M., Hareland, G.: Novel nanoparticle-based drilling fluid with improved characteristics. SPE international oilfeild nanotechnology conference, pp. 1–6. Society of Petroleum Engineers, Noordwijk (2012)
Acknowledgements
The author would like to appreciate the management of Covenant University for providing an enabling environment to carry out this research.
Funding
A specific grant from funding agencies in the public, commercial, or not-for-profit sectors was not received for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Afolabi, R.O. Enhanced oil recovery for emergent energy demand: challenges and prospects for a nanotechnology paradigm shift. Int Nano Lett 9, 1–15 (2019). https://doi.org/10.1007/s40089-018-0248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-018-0248-0