Abstract
The transparent conductive oxide CdO thick films are prepared by screen printing method followed by sintering route. The structural, optical and electrical properties of as-grown films are characterized by powder X-ray diffraction (XRD), scanning electron microscopy (SEM), atomic force microscopy (AFM), Fourier transform infrared (FTIR), ultraviolet–visible (UV–VIS) and DC conductivity measurement techniques. XRD, SEM and AFM studies reveal that the film deposited is polycrystalline, single phase and granular in nature. The crystallite size from XRD pattern’s most strong (111) peak calculated using Debye–Scherrer’s formula is 30 nm. IR transmission spectrum exhibits Cd–O stretching and bending mode peaks at 831 and 672 cm−1, respectively. Electrical properties were characterized by two-probe measurement in the temperature range between 300 and 400 K. Optical transmission spectroscopy and DC conductivity measurements have revealed the semiconducting nature of this film with direct band gap energy 2.53 and 0.29 eV activation energy and dc conductivity 4.5 × 10−7 (’Ωcm) −1.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Background
In recent years, transparent conductive oxide (TCO) layers have been drawing considerable attention for their potential applications in photovoltaic devices, gas sensors, phototransistors and other electronic and optoelectronic devices [1]. Cadmium oxide is one of the providential TCOs possessing high electric conductivity [2, 3], transmittance with moderate refractive index. It belongs to a class of n-type semiconductor oxide with a rock salt crystal structure having band gap energy in the interval of 2.2–2.8 eV. Optically, the films of cadmium oxide act as a selectively transmitting layer being transparent to the visible light and reflective to the thermal infrared radiation. CdO thick films have already been developed by various techniques such as spray pyrolysis [4], DC magnetron sputtering [5, 6], sol–gel [7, 8], pulsed laser deposition [9], sol–gel technique, etc. In this work, we have employed fast emerging screen printing technique to deposit CdO thick films.
Screen printing technique has been used as a multifaceted method for the fabrication of semiconductor layers in photovoltaic devices, especially II–IV compound semiconductors [10–12]. Compared to the other costly methods, screen printing is very simple, quicker, eco-friendly and provides a worthy method for film preparation on large area substrates with maximum utilization.
The aim of the present work is to deposit CdO thick films by screen printing technique and investigate their structural, optical and electrical properties to use them in optoelectronic device applications.
Methodology of materials
Merck-made Analytical Reagent (AR)-grade cadmium oxide (CdO), anhydrous cadmium chloride (CdCl2), ethylene glycol (C2H6O2, 99.99 % purity) are used for synthesis of CdO thick films by screen printing method.
Synthesis of CdO
For casting CdO thick film on glass substrate, CdO powder (99.999 % purity) was thoroughly mixed with anhydrous CdCl2 as adhesive agent and then ground in a mortar with ethylene glycol as binder to form thick paste. Before film deposition, the glass substrates were cleaned with acetone and deionised water and dried at 60 °C for 10 min in oven. The prepared paste was used in screen printer to deposit CdO thick film on glass substrate. The as-deposited film was heated at 110 °C for 2 h to remove volatile solvent and for better adherence of film to substrate [13]. This screen-printed film was further annealed in muffle furnace at 500 °C for 10 min to decompose organic compounds and achieve the desired stoichiometry of film [14].
The thickness of the film was measured by employing a profilometer (Surftest SJ-301) having the value of 1 µm. The steps involved in the preparation of CdO thick films by screen printing method as described above are presented in the following Fig. 1 schematic flow chart.
Characterization
Advanced Rigaku diffractometer was used to record X-ray diffraction (XRD) pattern in the 2θ range of 20°–60° using Cu–Kα X-ray radiation source. Profilometer (Surftest SJ-301) instrument was used for thickness measurement. The surface morphological information was derived using scanning electron microscope (SEM, Leo-440, UK). Atomic force microscopy (AFM) technique has been used to characterize the topography of CdO thick film. AFM images were recorded on Bruker Multimode 8-AM Atomic Force Microscope in (5 μm × 5 μm) and (50 μm × 50 μm) scanning area of the film. IR transmission spectrum was recorded by SHIMAZU-8400S, Japan Fourier transform infrared (FTIR) spectrophotometer in 4000–500 cm−1 range at ambient temperature with 4 cm−1 resolution. The optical transmission spectrum was recorded by Hitachi Spectrometer-3900 in the 350–1400 nm range. DC conductivity measurement was done using standard two-probe technique.
Results and discussions
Structure analysis
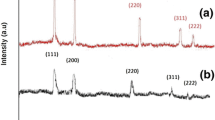
The purity and crystallinity of the sintered sample was examined using (XRD). The sharp intense peaks of CdO thick film confirmed good crystalline nature and besides no other impurity peak was seen, it suggested the formation of the single phase of CdO. The peaks originated at (111), (200), (220) reflections are shown in Fig. 2. The observed 2θ value of diffraction peaks, calculated and standard JCPDS card lattice parameters and crystallite size from Debye–Scherrer’s formula [15] are listed in Table 1. Debye–Scherrer’s formula used for calculation of crystallite size of CdO thick film is given in the following equation:
where D is the grain boundary size (in nm), λ is the X-ray wavelength β is the width (in radians) at half the maximum peak intensity, and θ is the Bragg angle. The crystallite size varies from 29 to 31 nm for these three planes.
The calculated lattice parameters match well with CdO (JCPDS) card No. 05-0640 reported data [16].
SEM analysis
Figure 3 shows scanning electron microscopy (SEM) micrograph of deposited thick film scanned by 15 kV electron beam at 5000× magnification. The SEM image reveals the polycrystalline, porous morphology with the inter-connected grains present on the film surface. The small crystallites agglomerated to form spindle, dumbbell and cuboidal-shaped particles and fused clusters are also seen in surface morphology of this film. Such structures may provide novel platform for photovoltaic, sensor and other device applications.
AFM analysis
AFM characterized the 2D and 3D topography of screen-printed CdO thick film, as shown in Fig. 4a, b, respectively. In Fig. 4a, the spherical grains are closely packed, uniformly distributed and amalgamation in some regions within the scanned area. While in Fig. 4b, 3D topography of the film exhibits columnar microstructure perpendicular to the surface. The mean surface roughness was measured to be ~70.00 nm. This surface roughness of CdO thick film reduces light reflection, but increases light absorption in the visible region of the solar spectrum in solar cells.
IR analysis
IR spectroscopy is a versatile tool to reveal structural details of bonding groups present through fundamental vibrations observed in mid-infrared region. The functional groups, molecular geometry and inter/intra-molecular interactions via hydrogen bonding are considered during IR transmission spectrum analysis [17]. Metal oxides generally give fingerprint absorption bands below 1000 cm−1 arising from inter-atomic stretching and bending vibrations. IR transmittance spectrum of CdO thick film recorded in 4000–600 cm−1 range is presented in Fig. 5. The spectrum exhibits broad transmittance band of water stretching mode with maximum at 3431 cm−1. The broadness of this mode confirms the presence of hydrogen bonding. C–H antisymmetric and symmetric stretching modes of methylene (CH2) group are observed at 2930 and 2821 cm−1, respectively. The presence of these modes generally arises from the organic compounds present in the atmosphere. In 2500–2000 cm−1 region, very complex vibrational rotational spectral lines of atmospheric carbon dioxide are obtained. Very strong peaks due to bending modes of CH2 appeared at 1372 cm−1. Cd–OH antisymmetric stretching mode as medium intensity peak is obtained at 1264 cm−1 while symmetric stretching components of Cd–OH mode appears at 1218 and 1164 cm−1. In addition to these peaks, the strong peak of Cd–O stretching mode and weak bending mode are obtained at 831 and 672 cm−1, respectively. These studies confirmed the formation of CdO film.
Optical properties
Figure 6 shows the optical transmission spectrum of CdO screen-printed film recorded in the 350–1400 nm range and the curve shows 80 % transmission in the visible region. At 400 nm, the transmission is ~10 % which starts increasing exponentially with sharp rise in 600 to 850 nm region and then at slow pace to 80 % at 1400 nm. Mostly, all II–VI semiconductors have direct band gaps. The relation between absorption coefficient (α) and incident photon energy (hν) can be written as [18]
where A is constant, E g is the energy separation between valence and conduction bands. The exponent n depends upon the quantum selection rules for particular material which is equal to 1/2 for direct band gap material. The photon energy (hν) for Y-axis can be calculated using Eq. (3).
where h is Planck’s constant (6.626 × 10−34 J/s), c is speed of light (3 × 108 m/s) and λ is wavelength of incident light.
Figure 7 shows the plot between (αhν)2 vs. hν, which is a straight line, indicating the direct transition. The energy band gap is obtained by extrapolating the linear part of the curve to zero absorption coefficient. The band gap is found to be 2.53 eV, which is in good agreement with earlier result [19].
Electrical properties
One of the reasons for the application of the CdO thin films in the optoelectronic devices technology is their good electrical conductivity even without any extrinsic doping [20]. The DC electrical conductivity measurements have been carried out in the temperature range 300–400 K. The electrical resistivity (ρ) was calculated using the Eq. (4) [21].
where ρ is the resistivity (Ωcm), A is the area, t is the thickness of film (cm), V is the measured voltage and I is the source current (A). To identify the nature of conduction process, activation energy has been calculated using the Arrhenius relation (5):
where σ is the conductivity \(({\frac{1}{\rho }})\), \(\sigma_0\) is the pre-exponential factor, \(\Delta E\) is the thermal activation energy for generation process, K is Boltzmann’s constant and T is the absolute temperature.
In Fig. 8, graph is plotted between \(\log (\sigma )\) and 103/T, to understand the charge conduction mechanism in CdO film. The variation of electrical conductivity with temperature indicates the semiconducting nature of the sample because conductivity increases with increase in operating temperature due to rise in electron carriers [22] in the film. The activation energy has been derived from the slope of the graph and it comes out to be 0.29 eV which is also in good agreement with the result reported earlier [23].
Conclusion
In conclusion, these investigations exhibit the successful utilization of a simple and economical screen printing method for preparing wide band gap semiconductor thick films. The structural, morphological, optical and electrical studies prove the suitability of these films for photovoltaic devices and other electronic applications. The XRD, SEM and AFM studies show that CdO films have polycrystalline nature with cubic structure, spherical grains and porous morphology. The direct energy band gap transition has been found to be 2.53 eV. FTIR spectrum reveals the formation of CdO with no trace of any type of impurity. DC conductivity measurement reveals the semiconducting nature of film and gives activation energy value 0.29 eV. This exhibits that the conduction process of carried charge is thermally activated. Thus, screen printing is a cost-effective and user-friendly technique and can be used to fabricate polycrystalline thick films having good stability and significant value of activation energy. Such types of films are suitable for solar cells and other optoelectronics devices.
References
Dakhel, A.: Optoelectronic properties of Eu- and H-codoped CdO films. Curr. Appl. Phys. 11, 11–15 (2011)
Yan, M., Lane, M., Kannewurf, C.R., Chang, R.P.H.: Selective-area atomic layer epitaxy growth of ZnO features on soft lithography-patterned substrates. Appl. Phys. Lett. 78, 2342–2344 (2001)
Ghosh, P.K., Das, S., Kundoo, S., Chattopadhyay, K.K.: Effect of fluorine doping on semiconductor to metal-like transition and optical properties of cadmium oxide thin films deposited by sol-gel process. J. Sol-Gel Sci. Technol. 34, 173–179 (2005)
Gandarilla, F., Morales-Acevedo, A., Vigil, O., Hesiquio-Garduo, V., Vaillant, L., Contreras-Puente, V.: Micro-structural characterization of annealed cadmium zinc oxide thin films obtained by spray pyrolysis. Mater. Chem. Phys. 78, 840–846 (2003)
Zhou, Q., Ji, Z., Hu, B.B., Chen, C., Zhao, L., Wang, C.: Low resistivity transparent conducting CdO thin films deposited by DC reactive magnetron sputtering at room temperature. Mater. Lett. 61, 531–534 (2007)
Ma, D., Ye, Z., Wang, L., Huang, J., Zhao, B.: Deposition and characteristics of CdO films with absolutely (200)-preferred orientation. Mater. Lett. 58, 128–131 (2003)
Ghosh, P.K., Das, S., Chattopadhyay, K.K.: Temperature dependent structural and optical properties of nanocrystalline CdO thin films deposited by sol–gel process. J. Nanopart. Res. 7, 219–225 (2005)
Santos-Cruz, J., Torres-Delgado, G., Castanedo-Perez, R., Jimenez-Sandoval, S., Jimenez-Sandoval, O., Zuniga-Romero, C.I., Marquez Marın, J., Zelaya-Angel, O.: Dependence of electrical and optical properties of sol–gel prepared undoped cadmium oxide thin films on annealing temperature. Thin Solid Films 493, 83–87 (2005)
Ismail, R.A., Rasheed, B.G., Salm, E.T., Al-Hadethy, M.: High transmittance–low resistivity cadmium oxide films grown by reactive pulsed laser deposition. J. Mater. Sci. Mater. Electron. 18, 1027–1030 (2007)
Kumar, V., Sharma, D.K., Bansal, M.K., Dwivedi, D.K., Sharma, T.P.: Synthesis and characterization of screen-printed CdS films. Sci. Sinter. 43, 335–341 (2011)
Zargar, R.A., Arora, M., Hafiz, A.K.: Investigation of physical properties of screen printed nanosized ZnO films for optoelectronic applications. Eur. Phys. J Appl. Phys. 70, 10403 (2015)
Zargar, R.A., Khan, S.D., Khan, M.S., Arora, M., Hafiz, A.K.: Synthesis and characterization of screen printed Zn0.97Cu0.03O thick film for semiconductor device applications. Phys. Res. Int. 2014, Article ID 464809, 5 pp. (2014)
Ismail, B., Abaab, M., Rezig, B.: Structural and electrical properties of ZnO films prepared by screen printing technique. Thin Solid Films 383, 92–94 (2001)
Sharma, M., Kumar, S., Sharma, L.M., Sharma, T.P., Husain, M.: CdS sintered films: growth and characteristics. Physica B 348, 15–20 (2004)
Cullity, B.D.: Elements of X-ray Diffractions. Addison-Wesley, Reading (1978)
Lanje, A.S., Ningthoujam, R.S., Sharma, S.J., Pode, R.B.: Luminescence and electrical resistivity properties of cadmium oxide nano particles. Indian J. Pure Appl. Phys. 49, 234–238 (2011)
Nandi, S.K., Chakraborty, S., Bera, M.K., Maiti, C.K.: Structural and optical properties of ZnO films grown on silicon and their application in MOS devices in conjunction with ZrO2 as a gate dielectric. Bull. Mater. Sci. 30, 247–254 (2007)
Tauc, J. (ed.): Amorphous and Liquid Semiconductors, vol. 159. Plenum Press, New York (1974)
Khallaf, H., Chen, C.-T., Chang, L.-B., Lupan, O., Dutta, A., Heinrich, H., Shenoud, A., Chowa, L.: Investigation of chemical bath deposition of CdO thin films using three different complexing agents. Appl. Surf. Sci. 257, 9237–9242 (2011)
Bhosale, C.H., Kambale, A.V., Kokate, A.V., Rajpure, K.Y.: Structural, optical and electrical properties of chemically sprayed CdO thin films. Mater. Sci. Eng. B 122, 67–71 (2005)
Singhal, S., Kaur, J., Namgyal, T., Sharma, R.: Cu-doped ZnO nanoparticles: synthesis, structural and electrical properties. Physica B 407, 1223–1226 (2012)
Ghosh, P.K., Maity, R., Chattopadhyay, K.K.: Electrical and optical properties of highly conducting CdO: F thin film deposited by sol–gel dip coating technique. Sol. Energy Mater. Sol. Cells 81, 279–289 (2004)
Uplane, M.D., Kshrisagar, P.N., Lokhande, B.J., Lokhande, C.D.: Preparation of cadmium oxide films by spray pyrolysis rand its conversation into chalcogenide films. Indian J. Pure Appl. Phys. 37, 616–619 (1999)
Acknowledgments
RAZ is thankful to the CSIR-NPL, New Delhi for giving permission to use characterization facilities required for analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Authors’ contributions
RAZ was involved in the acquisition, analysis, and interpretation of data. SC was involved in carried out optical and XRD characterization. MA was involved in analysis of FTIR spectrum of film and drafting the manuscript. AKZ revised the manuscript critically and gave final approval for submission. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zargar, R.A., Chackrabarti, S., Arora, M. et al. Synthesis, characterization and interpretation of screen-printed nanocrystalline CdO thick film for optoelectronic applications. Int Nano Lett 6, 99–104 (2016). https://doi.org/10.1007/s40089-015-0172-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0172-5