Abstract
The main purpose of this study is to explain the absorption of zinc from aqueous solution by grapheme oxide and functionalized grapheme oxide with glycine as the adsorbent surface. For confirmed functionalized graphene oxide, the glycine amino group was added to the surface of graphene oxide. The effects of the initial concentration of Zn(II) ions and contact time were studied. Results showed that with increasing initial concentration of Zn(II) ions, the adsorption capacity increased. The adsorption capacity did not show a large change after 50 min; therefore, for the study of kinetic parameters, the optimal time of 50 min was selected. The chemical structure of graphene oxide was confirmed by using FT-IR analysis. The adsorption process of Zn(II) ions graphene oxide and functionalized graphene oxide–glycine surfaces was fixed at 298 K and pH 6. The pseudo-first-order and the pseudo-second-order (types I, II, III and IV) kinetic models were tested for the adsorption process and the results showed that the kinetic parameters best fit type (I) of the pseudo-second-order model. A high R 2 was used to be the best match.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, heavy metal contamination in wastewater has become a major subject. Heavy metals in nature are hazardous to plants, animals, and humans even in slight concentrations. Therefore, the concentrations of ions of heavy metals solvable in effluents must be controlled well to prevent their toxicity effects. Chemists are trying to find ways to remove pollutants and contaminants. Among many heavy metals, zinc is one of the common pollutants in different industrial effluents. Zinc (ion) is usually found in industrial wastewater such as acid mine drainage, galvanizing plants, municipal and also natural materials. Many methods including physical and chemical methods, such as chemical precipitation [1], filter media [2], biosorption [3], flocculation [4] and ion exchange [5], have been used to solve this problem by removing heavy metals from wastewater. One of the most common methods is adsorption due to its easiness and simplicity. A study of the literature shows which diverse synthetic and natural adsorbents had been used for the adsorption of zinc from aqueous solution [6, 7]. However, adsorption is the maximum common, effective removal method, for many low-level pollutants.
Graphene (Gr), a single layer of sp2-hybridized carbon atoms in a closely packed honeycomb two-dimensional lattice, has attracted enormous attentions since its discovery in 2004 [8]. One of the most commonly used derivatives of graphene is graphene oxide (GO). One of the important structural elements of graphite is GO that is bound together by van der Waals forces. GO has attracted a lot attention because of its capability and usages in energy storage, electronics and water treatment membranes [9].
In this study, we investigated the adsorption of Zn(II) by graphene oxide and functionalized graphene oxide–glycine. Also, the effect of parameter contact time on Zn(II) ions adsorption was studied. For characterizing the surface of adsorbents, the FT-IR technique was used. Before and after the adsorption process, infrared spectra of the adsorbent and metal-loaded adsorbent samples were recorded in the range 4000 to 500 cm−1.
Experimental
Materials and methods
All experiments were conducted in a closed 250 mL pyramid glass bottle, and HCl or NaOH 0.1 M (provided by Merck Company with maximum purity) was used to adjust the pH of the solution. Zinc nitrate hexahydrate (reagent grade 98 %, CAS Number 10196-18-6, linear formula Zn(NO3)2·6H2O, molecular weight 297.49) was obtained from Sigma-Aldrich. Graphene oxide (composition: carbon (79 %), oxygen (20 %), flake size 0.5–5 µm, thickness 1 atomic layer—at least 80 %) was purchased from Supermarket USA. Glycine (CAS Number 56-40-6, impurities ≤0.01 % insoluble matter, pKa (25 °C) = 2.35, M.P = 240 °C), N,N-dimethylformamide (DMF, 99.9 %), 1-ethyl-(3-3-dimethylaminopropyl) carbodiimide (EDC, 99 %) and N-hydroxysuccinimide (NHS, 99.9 %) were obtained from Sigma-Aldrich.
FT-IR spectroscopy from Perkin Elmer-E100 Company was used within 400–4000 cm−1. At the end of the equilibrium period, the suspensions were centrifuged at 3500 rpm for 10 min, and the supernatant was then filtered through 0.2 μm filter paper (Gelmen Sciences) for later analysis of the atomic absorption spectrophotometry (AAS) (Perkin Elmer Analyst 700). The difference between the initial and the equilibrium Zn(II) ion concentration determines the amount of Zn(II) ion adsorbed on GO–G and GO as adsorbent surfaces. Each experiment was performed twice and experimental results were average values. Doubly distilled water was used and all adsorbents were washed before use. Ultrasonic bath (71020-DTH-E; Model 1510 DTH, 220V; EMS Company, Hatfield, PA) was used to mix the particles of GNPs and SWCNTs were used to aggregate and to form the bulk.
Preparation of GO and GO–G surfaces
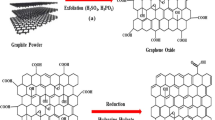
In this study, we synthesized graphene oxide–glycine (GO–G) powders via condensation reaction between graphene oxide (GO) powder and glycine and using FT-IR to characterize the presence of glycine on the GO surface as adsorbent.
GO (45 mg) was obtained by the reaction of 40 mg glycine in ethanol with the aid of coupling agents, EDC (50 mg) and NHS (25 mg), at 70 °C for 36 h. At the end of the reaction, GO–G–SH nanocomposite was then purified by filtering through a 0.22 µm membrane, followed by washing with copious amounts of ethanol and water [1:1 (v/v)] and then drying at 85 °C for 36 h. GO–G–SH nanocomposite surfaces were used as an adsorbent for Zn(II) ion removal from aqueous solution (Scheme 1).
Zn(II) ions adsorption study
For adsorption of zinc by graphene oxide and functionalized GO–G from aqueous solutions, 20 mg of adsorbents was added to 20 mL of Zn(II) ion solution with known initial concentration (15 ppm). The contact time of adsorption Zn(II) ions on graphene oxide and functionalized GO–G was confirmed by preliminary experiments to be 50 min at 298 K and pH 6. After 50 min, Zn(II) ions were removed from the solution by graphene oxide and functionalized GO–G. The bottles of solutions were placed in an ultrasonic that was operated at defined temperatures and times and the remaining concentration of Zn(II) ions was determined by atomic absorbance spectrophotometry AAS (Perkin Elmer A Analyst 700) to calculate the removal of Zn(II) ions by graphene oxide and functionalized GO–glycine using the following expression Eq. (1) [10]:
where q e (mg g−1) is the amount of Zn(II) ions taken up by the adsorbents, C 0 (mg L−1) represents the initial Zn(II) ion concentration, C e (mg L−1) the equilibrium concentration of the Zn(II) ions remaining in the solution, W (g) the adsorbent mass and V (L) the volume of the aqueous solution [11].
Kinetic experiments
For the determination of the effect of time on the adsorption of Zn(II) ions at an initial concentration of 15 ppm by graphene oxide and functionalized GO–G surfaces prepared Zinc samples by adding 20 mg of graphene oxide and functionalized GO–G adsorbents into 20 mL of Zn(II) ions aqueous solutions after a certain period of time at 10,20, 30, 40, 50, 60 and 70 min the samples were collected and the concentration of Zn(II) ions in the aqueous solutions were determined using the atomic absorbance spectrophotometry AAS (Perkin Elmer A Analyst 700) was measured (±0.01 %). These result s showed that the removal of zinc happened within the initial few minutes and the adsorbed quantity of Zn(II) ions reached its equilibrium amount very rapidly. The equilibrium time for adsorption of Zn(II) ions was about 50 min.
The Zn(II) ion adsorption capacity at time t (q t ), in mg/g, was then calculated using Eq. (2) [9]:
where C 0 (mg L−1) is the initial Zn(II) ion concentration, C t (mg L−1) the Zn(II) ion concentration at time t, W (g) the adsorbent mass and V (L) the volume of Zn(II) ion solution.
Characterization
The functional groups were characterized using a Perkin Elmer-E100, 2 m−1 resolution) Spectra were acquired in the 4000–400 cm−1 range (Fig. 3).
Result and discussions
Characterizations of adsorbents
FT-IR spectra of the graphene oxide and GO–G nanocomposite were obtained Fig. 1. The FT-IR spectrum is illustrated in Fig. 1. For graphene oxide, peaks at 3432 and 1711 cm−1 were related to –OH and C=O bands, respectively. Also, we can see aromatic C=C (1622 cm−1), carboxyl C–O (1414 cm−1), epoxy C–O (1228 cm−1) and C–O (1116 cm−1). After synthesis, nanocomposite GO–G, aliphatic C–H stretching vibrations at 2944 and 2889 cm−1 and an amide band at 1641 cm−1 in the FT-IR spectroscopy were seen [4]. TGA and SEM are presented (shown) in Figs. 2 and 3, respectively. With regard to TGA, the thermal gravimetric was increased on adding glycine to GO. The main reason related to the bonding between the carboxyl group (COOH) on the GO surface and amine in glycine is that we can see new bonding in the nanocomposite.
Contact time at removal of Zn(II) ions
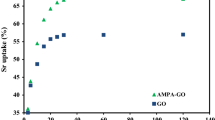
In the study of the adsorption process, one of the important factors is contact time. In this study, adsorption experiments occurred at several times: 10, 20, 30, 40, 50, 60 and 70 min. Figure 4 shows the effect of contact time on the adsorption of Zn(II) ions on graphene oxide and functionalized GO–G adsorbents. Results showed that the adsorption capacity increased with the increase in time. After 50 min, the changes in the removal of Zn(II) ions were very slow; therefore, 50 min was determined as optimum time for adsorption of Zn(II) ions on graphene oxide and functionalized GO–G adsorbents at 298 K and pH 6.
Effect of initial Zn(II) ion concentration
Adsorption of Zn(II) ions by graphene oxide and functionalized GO–G surfaces was performed at different initial concentrations of Zn(II) ions at 10, 15, 20 and 25 ppm, pH 6, temperature 298 K and 50 min. Figure 5 shows that with increasing the initial concentration of zinc ions from 10 to 25 mg L−1, the removal of Zn(II) ions was increased. The results indicate that there are higher attractive forces between Zn(II) ions and graphene oxide and functionalized GO–G adsorbents, because the surfaces of adsorbents would be as well besieged by (H3O+) ions which elevate ion interactions with binding sites of the surface of adsorbents [12].
Adsorption kinetics study
The kinetics of the adsorption process is a features that may strongly constrain the use of the adsorbent. The small kinetics adsorption significantly enlarges the removal time, which makes the adsorption inadequate [13]. In this study, we used the pseudo-first-order and four types of pseudo-second-order kinetic models to test the experimental data of removal of Zn(II) ions from aqueous solutions.
The pseudo-first-order kinetic model
The pseudo-first-order kinetic model is more suitable for low concentrations of solute [14]. The liner form of the pseudo-first-order equation is explained as follows [15]:
where q e (mg g−1) is the amounts of Zn(II) ions adsorbed on graphene oxide and functionalized GO–G equilibrium, q t (mg g−1) is the amount of Zn(II) ions adsorbed on graphene oxide and functionalized GO–G at time t, and k 1 is the rate constant, determined by plotting ln (q e − q t ) versus t (Fig. 6; Table 1).
The pseudo-second-order kinetic model
Ho presented a pseudo-second-order rate law expression in 1995 that explained how the rate depended on the adsorption equilibrium capacity but not on the concentration of the adsorbate [16]. In the adsorption process used, the change in concentration of an adsorbent or the adsorption unit time determined the adsorption rate. The kinetic rate equations can be explained as follows:
An integrated pseudo-second-order rate law can be obtained from Eq. (4) for the boundary conditions t = 0 to t = t and q t = 0 to q t = q t , and is given by [16]:
Equation (5) can be rearranged to obtain a linear form:
where t (min) is the adsorption time; q t (mg g−1) the amount of adsorbate at time t; q e (mg g−1) the amount of adsorbate at equilibrium; k 2 (g mg−1 min−1) the equilibrium rate constant of pseudo-second-order adsorption.
Four types of the pseudo-second-order models were defined as [17, 18]:
The results showed that the values of the correlation coefficients (R 2) for type (I) of the pseudo-second-order kinetic model are closest to 1 for both adsorbents, much higher than the correlation coefficients derived from the pseudo-first-order and type II, type III and type IV of the pseudo-second-order kinetic models. These results suggest that adsorption of Zn(II) ions on graphene oxide and functionalized GO–G adsorbents in compliance with type (I) of the pseudo-second-order kinetic model (Figs. 7, 8, 9, 10).
Conclusions
In this work, adsorption of Zn(II) ions by graphene oxide and functionalized GO–G were examined at 298 K and pH 6. Optimal time for the adsorption process was selected at 50 min, because we did not have a substantial increase in the adsorption capacity of Zn(II) ions on graphene oxide and GO–G after 50 min. The effect of the initial concentration of zinc ions in the aqueous solution was studied, and the results showed that the adsorption capacity was increased with increase in the initial concentration of Zn(II) ions. The pseudo-first-order and four types of the pseudo-second-order kinetic models were used to test the experimental data, and the results suggest that adsorption of Zn(II) ions on adsorbents followed type (I) of the pseudo-second-order kinetic model because of the higher value of (R 2).
References
Ren, X., Shao, D., Yang, S., Hu, J., Sheng, G., Tan, X., Wang, X.: Comparative study of Pb(II) sorption on XC-72 carbon and multi-walled carbon nanotubes from aqueous solutions. Chem. Eng. J. 170, 170–177 (2011)
Tian, Y., Gao, B., Morales, V.L., Wu, L., Wang, Y., Muñoz-Carpena, R., Cao, C., Huang, Q., Yang, L.: Methods of using carbon nanotubes as filter media to remove aqueous heavy metals. Chem. Eng. J. 210, 557–563 (2012)
Sharan-Liew, Y., Josephjand, C.G., HOW, S.: Biosorption of lead contaminated wastewater using cattails (Typhaangustifolia) leaves: kinetic studies. J. Serb. Chem. Soc. 76, 1037–1047 (2011)
Sadowski, Z.: Effect of biosorption of Pb(II), Cu(II) and Cd(II) on the zeta potential and flocculation of Nocardia sp. Miner. Eng. 14, 547–552 (2001)
Tarase, M.V., Zade, A.B., Gurnule, W.B., Resin, I.: Synthesis, characterization, and ion-exchange properties of terpolymer resins derived from 2,4-dihydroxypropiophenone, biuret, and formaldehyde. J. Appl. Polym. Sci. 108, 738–746 (2008)
Shrestha, S., Son, G., Lee, S., Lee, T.: Isotherm and thermodynamic studies of Zn(II) adsorption on lignite and coconut shell-based activated carbon fiber. Chemosphere 92, 1053–1061 (2013)
Al-Tohamia, F., Ackachab, M.A., Belaidc, R.A., Hamaadi, M.: Adsorption of Zn(II) ions from aqueous solutions by novel adsorbent: Ngella sativa seeds. Sciverse. Scien. Cedirect. 5, 400–404 (2013)
Ye, X., Du, Y., Lu, D., Wang, C.: Fabrication of cyclodextrin-coated poly (diallyldimethylammonium chloride)-functionalized graphene composite film modified glassy carbon-rotating disk electrode and its application for simultaneous electrochemical determination colorants of sunset yellow and tartrazine. Anal. Chim. Acta 779, 22–34 (2013)
Algothmi, W.M., Murthy Bandaru, N., Yu, Y., Shapter, J.G., Ellis, A.V.: Alginate–graphene oxide hybrid gel beads: an efficient copper adsorbent material. J. Colloid Interface Sci. 397, 32–38 (2013)
Wu, Q., Feng, C., Wang, C., Wang, Z.: A facile one-pot solvothermal method to produce superparamagnetic graphene–Fe3O4 nanocomposite and its application in the removal of dye from aqueous solution. Colloid Sur. B 101, 210–214 (2013)
Ai, L., Jiang, J.: Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene–carbon nanotube hybrid. Chem. Eng. J. 192, 156–163 (2012)
Machado, F.M., Bergmann, C.P., Fernandes, T.H.M., Lima, E.C., Royer, B., Calveteb, T., Faganc, S.B.: Adsorption of reactive red M-2BE dye from water solutions by multi-walled carbon nanotubes and activated carbon. J. Hazard Mater. 192, 1122–1131 (2011)
Ramana, D.K.V., Yu, J.S., Seshaiah, K.: Silver nanoparticles deposited multiwalled carbon nanotubes for removal of Cu(II) and Cd(II) from water: surface, kinetic, equilibrium, and thermal adsorption properties. Chem. Eng. J. 223, 806–815 (2013)
Zhang, X., Cheng, C., Zhao, J., Maa, L., Sun, S., Zhao, C.: Polyethersulfone enwrapped graphene oxide porous particles for water treatment. Chem. Eng. J 215, 72–81 (2013)
Leng, Y., Guo, W., Su, S., Yi, C., Xing, L.: Removal of antimony(III) from aqueous solution by graphene as an adsorbent. Chem. Eng. J. 211, 406–411 (2012)
Ho, Y.: Pseudo-isotherms using a second order kinetic expression constant. Adsorption 10, 151–158 (2004)
Robati, D.: Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 3, 55 (2013)
Dora, T.K., Mohanty, Y.K., Roy, G.K., Sarangi, B.: Adsorption studies of As(III) from wastewater with a novel adsorbent in a three-phase fluidized bed by using response surface method. J. Environ. Chem. Eng. 1, 150–158 (2013)
Acknowledgments
The author would like to thank the Islamic Azad, Roudehen Branch for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Najafi, F. Removal of zinc(II) ion by graphene oxide (GO) and functionalized graphene oxide–glycine (GO–G) as adsorbents from aqueous solution: kinetics studies. Int Nano Lett 5, 171–178 (2015). https://doi.org/10.1007/s40089-015-0151-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0151-x