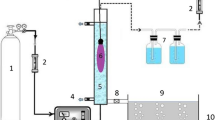
Abstract
Reactive dyes are widely consumed in the textile industrial sector due to their excellent properties, bright color, excellent color fastness, and ease of application; nevertheless, they are challenging to treat using existing conventional treatment methods due to their refractory and hazardous nature. In the present work, simulated reactive dye wastewater degradation was experimented with using Ozonation, O3/UV, and O3/UV/PS process. The experiments were carried out in a 3 L reactor for two different reactive dyes: reactive red 120 (RR120) and reactive yellow 145 (RY145), with their initial concentration ranges from 500 to 1500 mgL−1. The present study concludes that simple ozonation resulted in only 49% TOC removal, while O3/UV processes removed 57% TOC after 90 min of treatment. The highest efficiency was achieved in a coupled O3/UV/PS process with TOC removal of 88% at 66 W UV intensity, 1:40 TOC:PS ratio, 1.86 gh−1 ozone dose, and alkaline pH. It was also observed that the TOC removal was higher in RR120 compared to RY145. Finally, electrical energy per order was evaluated for the various ozone-based AOPs, and O3/UV/PS provided the best results with % TOC removal.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article.
References
J. Wang, H. Chen, R. Yuan, F. Wang, F. Ma, B. Zhou, Intensified degradation of textile wastewater using a novel treatment of hydrodynamic cavitation with the combination of ozone. J. Environ. Chem. Eng. (2020). https://doi.org/10.1016/j.jece.2020.103959
Mohammed, A E, H H Hamed, W M Sh Alabdraba, and O M Ali. 2019. "COD removal from disperse blue dye 79 in wastewater by using Ozone-Fenton process." IOP Conf. Series: Materials Science and Engineering. 1-9. doi:https://doi.org/10.1088/1757-899X/518/6/062015.
B. Lellis, C.Z. Fávaro-Polonio, J.A. Pamphile, J.C. Polonio, Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innovat 3, 275–290 (2019). https://doi.org/10.1016/j.biori.2019.09.001
S. Ledakowicz, M. Solecka, R. Zylla, Biodegradation, decolourisation and detoxification of textile wastewater enhanced by advanced oxidation processes. J. Biotechnol. 89, 175–184 (2001)
L. Bilinska, K. Blus, M. Foszpanczyk, M. Gmurek, S. Ledakowicz, Catalytic ozonation of textile wastewater as a polishing step after industrial scale electrocoagulation. J Environ Manage 265, 110502 (2020). https://doi.org/10.1016/j.jenvman.2020.110502
S. Alahiane, A. Sennaoui, F. Sakr, M. Dinne, S. Qourzal, A. Assabbane, Photo-mineralization of azo dye reactive yellow 145 in aqueous medium by TiO2-coated non-woven fibres. Mediterranean J Chem 10(2), 107–115 (2020). https://doi.org/10.13171/mjc10102002051208sa
J. Dutta, A. Ahmed, A study of azo dye reactive red 120 induced genotoxicity on allium cepa L. J. Chem. Pharm. Res. 8(12), 93–97 (2016)
A. Navaei, M. Yazdani, H. Alidadi, M. Dankoob, Z. Bonyadi, A. Dehghan, A. Hosseini, Biosorption of Reactive Red 120 dye from aqueous solution using Saccharomyces cerevisiae: RSM analysis, isotherms and kinetic studies. Desalin. Water Treat. 171, 418–427 (2019). https://doi.org/10.5004/dwt.2019.24780
A.L. Singh, S. Chaudhary, S. Kumar, A. Kumar, A. Singh, A. Yadav, Biodegradation of Reactive Yellow-145 azo dye using bacterial consortium: A deterministic analysis based on degradable Metabolite, phytotoxicity and genotoxicity study. Chemosphere 300, 134504 (2022). https://doi.org/10.1016/j.chemosphere.2022.134504
S. Krishnasamy, B.A. SaiAtchyuth, G. Ravindiran, B. Subramaniyan, M. Ramalingam, J.U.B.S. Vamsi, B. Ramesh, N.A. Razack, Effective removal of reactive yellow 145 (RY145) using biochar derived from groundnut shell. Adv. Mater. Sci. Eng. (2022). https://doi.org/10.1155/2022/8715669
K.-T. Chung, Azo dyes and human health: A review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 34(4), 233–261 (2016). https://doi.org/10.1080/10590501.2016.1236602
L. Pandian, R. Rajasekaran, P. Govindan, Nanophotocatalytic ozonation of textile dyeing wastewater using Cu-ZnO nanocatalyst and study of reactor influencing parameters. Orient J Chem 35(1), 384–390 (2019)
N. Azbar, T. Yonar, K. Kestioglu, Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 55, 35–43 (2004). https://doi.org/10.1016/j.chemosphere.2003.10.046
F. Nilsson, Application of ozone in wastewater treatment: Oxidation of pharmaceuticals and filamentous bulking sludge (Department of Chemical Engineering, Lund University, Lund, 2017)
O.S.G. Soares, J.J. Orfao, D. Portela, A. Vieira, M.F.R. Pereira, Ozonation of textile effluents and dye solutions under continuous operation: Influence of operating parameters. J Hazardous Mater 137(3), 1664–1673 (2006). https://doi.org/10.1016/j.jhazmat.2006.05.006
L. Bilinska, M. Gmurek, S. Ledakowicz, Application of advanced oxidation technologies for decolorization and mineralization of textile wastewaters. J. Adv. Oxid. Technol 18(2), 185–194 (2015)
A.M. Soubh, M. Baghdadi, M.A. Abdoli, B. Aminzadeh, Activation of persulfate using an industrial iron-rich sludge as an efficient nanocatalyst for landfill leachate treatment. Catalysts 8(5), 218 (2018). https://doi.org/10.3390/catal8050218
Mo. Li, Q. Wen, Z. Chen, Y. Tang, B. Yang, Comparison of ozonation and UV based oxidation as pre-treatment process for ultrafiltration in wastewater reuse: Simultaneous water risks reduction and membrane fouling mitigation. Chemosphere 244, 125449 (2019). https://doi.org/10.1016/j.chemosphere.2019.125449
S. Sharma, N.P. Chokshi, J.P. Ruparelia, Comparative studies for the degradation of Reactive Black 5 dye employing ozone-based AOPs. Nanotechnology for Environmental Engineering 7(1), 9 (2022). https://doi.org/10.1007/s41204-021-00180-7
E. Mena, A. Rey, F.J. Beltrán, TiO2 photocatalytic oxidation of a mixture of emerging contaminants: A kinetic study independent of radiation absorption based on thedirect-indirect model. Chem. Eng. J. 339, 369–380 (2018). https://doi.org/10.1016/j.cej.2018.01.122
Nikita P. Chokshi, Jayesh P. Ruparelia. 2021. "Synthesis of Nano Ag-La-Co Composite Metal Oxide for Degradation of RB 5 Dye Using Catalytic Ozonation Process." Ozone: Science and Engineering 1-14. https://doi.org/10.1080/01919512.2021.1901070
J. Lee, U. von Gunten, J.-H. Kim, Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 54(6), 3064–3081 (2020). https://doi.org/10.1021/acs.est.9b07082
S. Sandip, R. Jayesh, Comparative study of ozone-based AOPs for degradation of Reactive Red 120. Res. J. Chem. Environ 26(2), 41–46 (2022)
A.R. Quaff, S. Venkatesh, K. Venkatesh, Degradation of azo dye by ozone oxidation: cost analysis and buffering effects on dye decomposition. Natl. Acad. Sci. Lett. 44, 339–341 (2021). https://doi.org/10.1007/s40009-020-01008-9
B. Adiraju, A.K. Saroha, Kinetics of ozone oxidation of acid red 131 monoazo dye in aqueous solution. J. Hazard. Toxic Radioact. Waste 21, 1–5 (2016)
C.Z. Abidin, M.R. Azner, O.-A. Fahmi, S.N.N.M. Makhtar, N.R. Rahmat, Decolourization of an azo dye in aqueous solution by ozonation in a semi-batch bubble column reactor. ScienceAsia 41, 49–54 (2015)
S. Wijannarong, S. Aroonsrimorakot, P. Thavipoke, A. Kumsopa, S. Sangjan, Removal of reactive dyes from textile dyeing industrial effluent by ozonation process. APCBEE Proc. 5, 279–282 (2013)
K. Turhan, S. Ilknur Durukan, A. Ozturkcan, Z. Turgut, Decolorization of textile basic dye in aqueous solution by ozone. Dyes Pigm. 92, 897–901 (2012)
E. Kusvuran, O. Gulnaz, A. Samil, Ö. Yildirim, Decolorization of malachite green, decolorization kinetics and stoichiometry of ozone-malachite green and removal of antibacterial activity with ozonation processes. J. Hazard. Mater. 186, 133–143 (2011)
A.R. Tehrani-Bagha, N.M. Mahmoodi, F.M. Menger, Degradation of a persistent organic dye from colored textile wastewater by ozonation. Desalination 260, 34–38 (2010)
M. Tokumura, T. Katoh, Y. Kawase, Dynamic modeling and simulation of ozonation in a semibatch bubble column reactor: Decolorization and mineralization of azo dye orange II by ozone. Ind. Eng. Chem. Res. 48, 7965–7975 (2009)
M. Sundrarajan, G. Vishnu, K. Joseph, Ozonation of light-shaded exhausted reactive dye bath for reuse. Dyes Pigm. 75, 273–278 (2007)
K. Swaminathan, K. Pachhade, S. Sandhya, Decomposition of a dye intermediate, (H-acid) 1 amino-8-naphthol-3,6 disulfonic acid in aqueous solution by ozonation. Desalination 186, 155–164 (2005)
F. Zhang, A. Yediler, X. Liang, A. Kettrup, Effects of dye additives on the ozonation process and oxidation by-products: a comparative study using hydrolyzed C.I. Reactive Red 120. Dyes Pigm. 60, 1–7 (2004)
I. Arslan, I.A. Balcioǧlu, D.W. Bahnemann, Advanced chemical oxidation of reactive dyes in simulated dyehouse effluents by ferrioxalate-Fenton/UV-A and TiO2/UV-A processes. Dyes Pigments 47, 207–218 (2000). https://doi.org/10.1016/S0143-7208(00)00082-6
M.G. Abrile, M.L. Fiasconaro, M.E. Lovato, Optimization of reactive blue 19 dye removal using ozone and ozone/UV employing response surface methodology. SN Applied Sci 2(5), 995 (2020). https://doi.org/10.1007/s42452-020-2824-y
A. Bharadwaj, A.K. Saroha, Treatment of synthetic dye solution containing acid red 131 dye by ozonation. Int. J. ChemTech Res. 5, 688–693 (2013)
B. Cuiping, X. Xianfeng, G. Wenqi, F. Dexin, X. Mo, Ge. Zhongxue, Xu. Nian, Removal of rhodamine B by ozone-based advanced oxidation process. Desalination 278, 84–90 (2011)
H. Khan, N. Ahmad, A. Yasar, R. Shahid, Advanced oxidative decolorization of red Cl-5B: Effects of dye concentration, process optimization and reaction kinetics. Polish J. of Environ. Stud. 19(1), 83–92 (2010)
C.-H. Wu, H.-Y. Ng, Degradation of C.I. reactive red 2 (RR2) using ozone-based systems: Comparisons of decolorization efficiency and power consumption. J. Hazard. Mater. 152, 120–127 (2008)
H.-J. Hsing, E.-E. Pen-Chi, C. Chang, M.-Y. Chen, The decolorization and mineralization of Acid Orange 6 azo dye in aqueous solution by advanced oxidation processes: A comparative study. J. Hazard. Mater. 141, 8–16 (2007)
Liu BW, Chou MS, Kao CM, & Huang BJ (2004) Evaluation of selected operational parameters for the decolorization of dye-finishing wastewater using UV/ozone. Ozone: Science and Engineering, 26(3): 239-245.
X. Liu, L. Wen, C. Wang, Le. Zhang, A. Zhang, Treatment of dinitrodiazophenol industrial wastewater by an ozone/persulfate process. Desalin. Water Treat. 198, 224–231 (2020). https://doi.org/10.5004/dwt.2020.25913
A. Amr, S. Salem, H.A. Aziz, M.J.K. Bashir, S.Q. Aziz, T.M. Alslaibi, Comparison and optimization of ozone–based advanced oxidation processes in the treatment of stabilized landfill leachate. J Eng Res Technol 2(2), 122–130 (2015)
A. Amr, S. Salem, H.A. Aziz, M.N. Adlan, M.J.K. Bashir, Pretreatment of stabilized leachate using ozone/persulfate oxidation process. Chem Eng J 221, 492–499 (2013)
X. Yu, Q. Wenlei, X. Yuan, L. Sun, F. Pan, D. Xia, Synergistic mechanism and degradation kinetics for atenolol elimination via integrated UV/ozone/peroxymonosulfate process. J. Hazard. Mater. (2020). https://doi.org/10.1016/j.jhazmat.2020.124393
J.R. Bolton, K.G. Bircher, W. Tumas, C.A. Tolman, Figures-of-merit for the technical development and application of advanced oxidation processes. J Adv Oxidat Technol 1(1), 13–17 (1996)
J.R. Bolton, K.G. Bircher, W. Tumas, C.A. Tolman, Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric-and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 73(4), 627–637 (2001)
N.N. Mahamuni, Y.G. Adewuyi, Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: a review with emphasis on cost estimation. Ultrason. Sonochem. 17(6), 990–1003 (2010)
O.C. Olatunde, A.T. Kuvarega, D.C. Onwudiwe, Photo enhanced degradation of contaminants of emerging concern in waste water. Emerging Contaminants 6, 283–302 (2020). https://doi.org/10.1016/j.emcon.2020.07.006
Z. Liu, K. Demeestere, S. Van Hulle, Comparison and performance assessment of ozone-based AOPs in view of trace organic contaminants abatement in water and wastewater: A review. J Environ Chem Eng 9(4), 105599 (2021). https://doi.org/10.1016/j.jece.2021.105599
A.H. Hilles, S.S. Abu, R.A. Amr, O.D. Hussein El-Sebaie, A.I. Arafa, Performance of combined sodium persulfate/H2O2 based advanced oxidation process in stabilized landfill leachate treatment. J. Environ. Manage. 166, 493–498 (2016). https://doi.org/10.1016/j.jenvman.2015.10.051
K. Tong, Z. Zhang, A. Lin, Q. Song, G. Ji, D. Wang, M. Zhang, Treatment of super heavy oil wastewater by a combined process of lignite-activated coke adsorption and immobilized biological filter degradation: performance and the relevant microbial community analysis. J. Chem. Technol. Biotechnol. 93(10), 2942–2951 (2018). https://doi.org/10.1002/jctb.5650
L. Chen, Y. Xu, Y. Sun, Combination of coagulation and ozone catalytic oxidation for pretreating coking wastewater. Int J Environ Res Public Health 16(10), 1705 (2019). https://doi.org/10.3390/ijerph16101705
Z. Yang, Y. Zhang, W. Zhu, X. Zan, L. Zhang, Y. Liu, Effective oxidative degradation of coal gasification wastewater by ozonation: A process study. Chemosphere 255, 126963 (2020)
Hilles AH, AMR, SSA, Alkarkhi AF, & Hossain MS (2019) The effect of persulfate oxidation on the biodegradability of concentrated anaerobic stabilized leachate. Sains Malaysiana, 48(11), 2381-2390https://doi.org/10.17576/jsm-2019-4811-09.
F. Ali, J.A. Khan, N.S. Shah, M. Sayed, H.M. Khan, Carbamazepine degradation by UV and UV-assisted AOPs: Kinetics, mechanism and toxicity investigations. Process Saf. Environ. Prot. 117, 307–314 (2018). https://doi.org/10.1016/j.psep.2018.05.004
L. Bilinska, M. Gmurek, S. Ledakowicz, Comparison between industrial and simulated textile wastewater treatment by AOPs–Biodegradability, toxicity and cost assessment. Chem. Eng. J. 306, 550–559 (2016). https://doi.org/10.1016/j.cej.2016.07.100
A.C. Mecha, M.S. Onyango, A. Ochieng, C.J.S. Fourie, M.N.B. Momba, Synergistic effect of UV–vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study. J. Catal. 341, 116–125 (2016). https://doi.org/10.1016/j.jcat.2016.06.015
M.E. Lovato, M.B. Gilliard, A.E. Cassano, C.A. Martín, Kinetics of the degradation of n-butyl benzyl phthalate using O3/UV, direct photolysis, direct ozonation and UVeffects. Environ Sci Pollut Res (2014). https://doi.org/10.1007/s11356-014-2796-9
S. Gurudev, S. Subramaniam, F. Chiampo, UV light-irradiated photocatalytic degradation of coffee processing wastewater using TiO2 as catalyst. Environments 7(47), 1–13 (2020). https://doi.org/10.3390/environments7060047
M.A. Hassaan, A. El Nemr, F.F. Madkour, Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. Egypt. J. Aquat. Res. 43, 11–19 (2017). https://doi.org/10.1016/j.ejar.2016.09.006
G.G. Bessegato, J.C. Cardoso, B.F. Da Silva, M.V.B. Zanoni, Combination of photoelectrocatalysis and ozonation: A novel and powerful approach applied in acid yellow 1 mineralization. Appl Catalysis B: Environ 180, 161–168 (2016). https://doi.org/10.1016/j.apcatb.2015.06.013
N.P. Chokshi, D. Patel, R. Atkotiya, J. Ruparelia, Catalytic ozonation of reactive black 5 in aqueous solution over a La-Co-O catalyst. J Indian Chem Soc 97, 373–378 (2020)
Chokshi, N. P., & Ruparelia, J. P. (2020). Catalytic Ozonation of Reactive Black 5 Over Silver–Cobalt Composite Oxide Catalyst. Journal of The Institution of Engineers (India): Series A, 101, 433-443. https://doi.org/10.1007/s40030-020-00454-4.
Kerwin, Rakness. 2011. Ozone in Drinking Water Treatment: Process Design, Operation, and Optimization. American water works association.
J.C. Cardoso, G.G. Bessegato, M.V. Boldrin Zanoni, Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 98, 39–46 (2016). https://doi.org/10.1016/j.watres.2016.04.004
A. Ikhlaq, M. Zafar, F. Javed, A. Yasar, A. Akram, S. Shabbir, F. Qi, Catalytic ozonation for the removal of reactive black 5 (RB-5) dye using zeolites modified with CuMn2O4/gC3N4 in a synergic electro flocculation-catalytic ozonation process. Water Sci. Technol. 84(8), 1943–1953 (2021). https://doi.org/10.2166/wst.2021.404
B. Bakheet, S. Yuan, Z. Li, H. Wang, J. Zuo, S. Komarneni, Y. Wang, Electro-peroxone treatment of Orange II dye wastewater. Water Res 47(16), 6234–6243 (2013)
M.E. Lovato, C.A. Martín, A.E. Cassano, A reaction kinetic model for ozone decomposition in aqueous media valid for neutral and acidic pH. Chem Eng J 146(3), 486–497 (2009)
K. Krawczyk, S. Wacławek, E. Kudlek, D. Silvestri, T. Kukulski, K. Grübel, M. Černík, UV-catalyzed persulfate oxidation of an anthraquinone based dye. Catalysts 10(4), 456 (2020). https://doi.org/10.3390/catal10040456
C.V. Rekhate, J.K. Srivastava, Recent advances in ozone-based advanced oxidation processes for treatment of wastewater-A review. Chem Eng J Adv 3, 100031 (2020). https://doi.org/10.1016/j.ceja.2020.100031
E. Adar, Removal of acid yellow 17 from textile wastewater by adsorption and heterogeneous persulfate oxidation. Int J Environ Sci Technol 18, 483–498 (2021). https://doi.org/10.1007/s13762-020-02986-5
D.A. House, Kinetics and mechanism of oxidations by peroxydisulfate. Chem. Rev. 62(3), 185–203 (1962). https://doi.org/10.1021/cr60217a001
Y. Deng, C.M. Ezyske, Sulfate radical-advanced oxidation process (SR-AOP) for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate. Water Res. 45(18), 6189–6194 (2011). https://doi.org/10.1016/j.watres.2011.09.015
Y. Pan, Y. Zhang, M. Zhou, J. Cai, Y. Tian, Enhanced removal of emerging contaminants using persulfate activated by UV and pre-magnetized Fe0. Chem Eng J 361, 908–918 (2019). https://doi.org/10.1016/j.cej.2018.12.135
J. Yang, M. Zhu, D.D. Dionysiou, Review - what is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. (2021). https://doi.org/10.1016/j.watres.2020.116627
Abidin, Azner Che Zulzikrami, Muhammad Ridwan Fahmi, Soon An Ong, Ibrahim Abdul Haqi, Sabri Siti Nasuha, and Razali Nur Aqilah. (2019) Study of O3/S2O82- Advanced Oxidation Processes (AOPs) for Removal of Dye Industrial Effluents. MATEC Web of Conferences 255. doi:https://doi.org/10.1051/matecconf/201925503003.
K. Kestioğlu, T. Yonar, N. Azbar, Feasibility of physico-chemical treatment and Advanced Oxidation Processes (AOPs) as a means of pretreatment of olive mill effluent (OME). Process Biochem. 40, 2409–2416 (2005). https://doi.org/10.1016/J.PROCBIO.2004.09.015
Acknowledgements
The authors would like to acknowledge the management of Nirma University for providing all the financial help to carry out the experimental work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SS: Data acquisition, Experimentation, Interpretation, NC: Data acquisition, Experimentation, Interpretation, JR: Conceptualization, Visualization, Supervision. All authors contributed in preparation of the manuscript, they have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Authors give copyrights to the journal as per the rules.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, S., Chokshi, N. & Ruparelia, J.P. Effect of Operating Parameters on O3, O3/UV, O3/UV/PS Process Using Bubble Column Reactor for Degradation of Reactive Dyes. J. Inst. Eng. India Ser. A 104, 565–578 (2023). https://doi.org/10.1007/s40030-023-00735-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40030-023-00735-8