Abstract
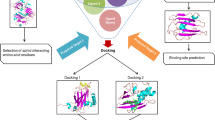
Viruses are causative agents of infections in plants which are found worldwide and cause a serious threat to modern agriculture. Since the effect of viral infections is decreased by reducing the population of the vector by spraying the pesticides, they have serious effects on human beings and the ecosystem. The objective of the present study was to evaluate the potential of natural plant product carvacrol as an anti-phytoviral agent against cauliflower mosaic virus (CaMV) with the proposed mode of action. In the present study, in vitro and in vivo inhibitory effect of carvacrol was examined in comparison with a roundup at varying concentrations, against the CaMV. It was purified from the infected leaf of Brassica oleracea. In addition, the inhibitory mechanism of carvacrol was studied after docking the carvacrol with P2 and P3 protein of CaMV virus using AutoDock 4.2 software. It was found that out of different concentrations of carvacrol (1 µl, 1.5 µl, 2 µl, 2.5 µl and 3 µl) tested in vitro and in vivo, 3 µl resulted 85.1% and 75% inhibition in comparison with roundup which showed 76.2% and 70%, respectively. Molecular docking showed an inhibitory mechanism of carvacrol against CaMV by effective binding with P3 protein in the pocket defined by chain C and chain A. The present study concluded the anti-phytoviral activity of carvacrol against CaMV by retarding its growth as a result of inhibiting P3 protein.







Similar content being viewed by others
References
Zhao L, Feng C, Wu K, Chen W, Chen Y, Hao X, Wu Y (2017) Advances and prospects in biogenic substances against plant virus: a review. Pestic Biochem Physiol 135:15–26
Elena SF, Fraile A, García-Arenal F (2014) Evolution and emergence of plantviruses. Adv Virus Res 88:161–191
Schreinemachers P, Balasubramaniam S, Boopathi NM, Ha CV, Kenyon L, Praneetvatakul S, Wu MH (2015) Farmers’ perceptions and management of plantviruses in vegetables and legumes in tropical and subtropical Asia. Crop Prot 75:115–123
Rothnie HM, Chapdelaine Y, Hohn T (1994) Pararetroviruses and retroviruses: a comparative review of viral structure and gene expression strategies. Adv Virus Res 44(C):1–67
Shibuya N, Minami E (2001) Oligosaccharide signalling for defence responses inplant. Physiol Mol Plant Pathol 59(5):223–233
Martinière A, Zancarini A, Drucker M (2009) Aphid transmission of cauliflower mosaic virus: the role of the host plant. Plant Signal Behav 4(6):548–550
Hoh F, Uzest M, Drucker M, Plisson-Chastang C, Bron P, Blanc S, Dumas C (2010) Structural insights into the molecular mechanisms of cauliflower mosaic virus transmission by its insect vector. J Virol 84(9):4706–4713
Laliberté J-F, Sanfaçon H (2010) Cellular remodeling during plant virus infection. Annu Rev Phytopathol 48(1):69–91
Khelifa M, Massé D, Blanc S, Drucker M (2010) Evaluation of the minimal replication time of Cauliflower mosaic virus in different hosts. Virology 396(2):238–245
Islam W, Qasim M, Noman A, Tayyab M, Chen S, Wang L (2018) Management of tobacco mosaic virus through natural metabolites. Rec Nat Prod 12(5):403–415
Hassan SA, Soleimani T (2016) Improvement of artemisinin production by different biotic elicitors in Artemisia annua by elicitation-infiltration method. Banat’s J Biotechnol 7(13):82–94
Armaka M, Papanikolaou E, Sivropoulou A, Arsenakis M (1999) Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir Res 43(2):79–92
Pimentel D, Burgess M (2014) Environmental and economic costs of the application of pesticides primarily in the United States. Integr Pest Manag 3:47–71
Hossain MM, Jahan I (2015) Azospirillum as biofertilizer and Bangladesh perspective. Banat’s J Biotechnol 6(11):69–82
Villa TG, Veiga-Crespo P (2014) Antimicrobial compounds: current strategies and new alternatives. In: Antimicrobial compounds: current strategies and new alternatives. Springer Publication, ISBN: 978-3-642-40-443-6
Chen J, Yan XH, Dong JH, Sang P, Fang X, Di YT, Hao XJ (2009) Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J Agric Food Chem 57(15):6590–6595
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343(6257):425–430
Holstein SA, Hohl RJ (2004) Isoprenoids: remarkable diversity of form and function. Lipids 39(4):293–309
Bezić N, Šamanić I, Dunkić V, Besendorfer V, Puizina J (2009) Essential oil composition and internal transcribed spacer (ITS) sequence variability of four South Croatian Satureja species (Lamiaceae). Molecules 14(3):925–938
Sivropoulou A, Papanikolaou E, Nikolaou C, Kokkini S, Lanaras T, Arsenakis M (1996) Antimicrobial and cytotoxic activities of Origanum Essential oils. J Agric Food Chem 44:1202–1205
Nóbrega RDO, Teixeira APDC, Oliveira WAD, Lima EDO, Lima IO (2016) Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm Biol 54(11):2591–2596
Valente JDSS, da Silva Fonseca ADO, Denardi LB, Dal Ben VS, de Souza Maia Filho F, Baptista CT, Pereira DIB (2016) In Vitro susceptibility of Pythium insidiosum to Melaleuca alternifolia, Mentha piperita and Origanum vulgare essential oils combinations. Mycopathologia 181(7–8):617–622
Lu M, Han Z, Xu Y, Yao L (2013) In vitro and in vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. J Microbiol Biotechnol 23(6):771–778
Shaaya E, Ravid U, Paster N, Juven B, Zisman U, Pissarev V (1991) Fumiganttoxicity of essential oils against four major stored-product insects. J Chem Ecol 17(3):499–504
Faria JMS, Sena I, Ribeiro B, Rodrigues AM, Maleita CMN, Abrantes I, da Silva Figueiredo AC (2016) First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J Pest Sci 89(1):207–217
Dunkic V, Bezic N, Vuko E, Cukrov D (2010) Antiphytoviral activity of Satureja montana L. ssp. variegata (host) P. W. Ball essential oil and phenol compounds on CMV and TMV. Molecules 15(10):6713–6721
Mohammadhassan R, Esfahani K, Kashefi B (2018) Constructional and functional evaluation of two new plant expression vectors-pBI121(GUS-6) and pBI121(5 + 1). Banat’s J Biotechnol 9(17):60–68
Kumar A, Senapati BK (2015) Genetic analysis of character association for polygenic traits in some recombinant inbred lines (ril’s) of rice (Oryza sativa L). Banat’s J Biotechnol 6(11):90–99
Ajay, Murcko MA (1995) Computational methods to predict binding free energy in ligand-receptor complexes. J Med Chem 38(26):4953–4967
Acknowledgments
The authors acknowledge the support of Lovely Professional University to provide the facilities to conduct this work smoothly.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement The present study signifies the efficacy of carvacrol, a natural plant product from Origanum vulgare, as an anti-phytovirus compound. The study also delineated the proposed action mechanism of carvacrol against CaMV virus by using computational biology. An effort was made to explore green approach to manage CaMV.
Rights and permissions
About this article
Cite this article
Bansal, A., Jan, I. & Sharma, N.R. Anti-phytoviral Activity of Carvacrol vis-a-vis Cauliflower Mosaic Virus (CaMV). Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 981–988 (2020). https://doi.org/10.1007/s40011-020-01166-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-020-01166-2