Abstract
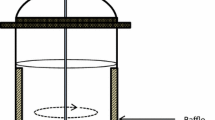
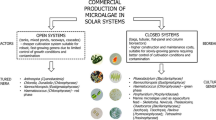
Development of photo-bioreactors (PBRs) has been accelerated owing to their potential application in the production of algal biofuel. But the application of PBRs should be looked beyond fuel and towards feed items/wastewater treatment solutions it can provide. The PBRs discussed in the review are broadly divided into two major categories, that is, open and closed type. The open PBRs are simple open pond systems while closed ones include vertical, flat plate, tubular, foil and recently developed porous substrate bioreactor. The efficiency of all PBRs depends on the surface-to-volume illumination ratio apart from mixing. The recently developed porous substrate bioreactor is technologically superior to its predecessors in terms of water usage and does not require biomass separation. The authors emphasize on the development of PBRs with the hope that their success would match as that of solar energy panels for power generation which have come a long way from the laboratory to real-world applications.








Similar content being viewed by others
References
Hsiun DY, Wu WT (1995) Mass transfer and liquid mixing in an Airlift reactor with a net draft tube. Bioproc Eng 12:221–225
Haque MW, Nigam KDP (1986) Optimum gas sparger design for bubble columns with a low height-to-diameter ratio. Chem Eng J 33:63–69
Rigonspier M, Vandenberghe LP, Medeiros ABP, Soccol CR (2011) Application of different types of bioreactors in bioprocesses. Nova Science Publishers Inc, NewYork, pp 55–90
Gupta D, Singh A, Sharma A, Nigam A (2013) Processing of bio-fuels. Wiley, Austin
Mueller-Rees C, Spruederer F, Klaus A (2012) Application publication multi-chamber photobioreactor. United States Patent Number US 2012/0202290
Borowitzka MA (1998) Algae as food. In: Wood BJB (ed) Microbiology of fermented foods. Springer, Boston, MA, pp 585–602
Couteau C, Coiffard L (2016) Seaweed application in cosmetics in seaweed in health and disease prevention. Academic Press, Cambridge, pp 423–441
Austin C, Derek Stewart D, Allwood JW, McDougall GJ (2018) Extracts from the edible seaweed, Ascophyllum nodosum inhibit lipase activity in vitro: contributions of phenolic and polysaccharide components. Food Funct 9:502–510
Chen X, Zhu X, Li R, Yao H, Lu Z, Yang X (2012) Photosynthetic toxicity and oxidative damage induced by nano-Fe3O4 on chlorella vulgaris in aquatic environment. Open J Ecol 2:21–28
Rupérez P (2002) Mineral content of edible marine seaweeds. Food Chem 79:23–26
Kumar S, Gupta R, Kumar G, Sahoo D, Kuhad RC (2013) Bioethanol production from Gracilaria verrucosa, a red alga, in a bio-refinery approach. Bio-resour Technol 135:150–156
Plaza M, Cifuentes A, Ibanez E (2008) In the search of new functional food ingredients from algae. Trends Food Sci Technol 19:31–39
Rosenberg G, Ramus J (1982) Ecological growth strategies in the seaweeds Gracilaria foliifera (Rhodophyceae) and Ulva sp. (Chlorophyceae): photosynthesis and antenna composition. Mar Ecol Prog Ser 8:233–241
Shimamatsu H (2004) Mass production of Spirulina, an edible microalga. Hydrobiologia 512:39–44
Lüning K, Mortensen L (2015) European aquaculture of sugar kelp (Saccharina latissima) for food industries: Iodine content and epiphytic animals as major problems. Bot Mar 58:449–455
Bito T, Teng F, Watanabe F (2017) Bioactive compounds of edible purple Laver Porphyra sp (Nori). J Agric Food Chem 65:10685–10692
Galland-Irmouli AV, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki JP, Villaume C, Guéant JL (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10:353–359
Coppen JW, Nambiar P (1991) Agar and alginate production from seaweed in India. Bay of Bengal programme (FAO United Nations), Madras, India
Necas J, Bartosikova L (2013) Carrageenan: a review. Vet Med 58:187–205
Parsaeimehr A, Lutzu GA (2016) Algae as a novel source of antimicrobial compounds: current and future perspectives. In: Kon K, Rai K (eds) Antibiotic resistance, 1st edn. Elsevier, London, pp 377–396
Gamal AAE (2010) Biological importance of marine algae. Saudi Pharm J 18:1–25
Garcia-Malea MC, Acien FG, Del Rio E, Fernandez JM, Ceron MC, Guerrero MG, Molina-Grima E (2009) Production of astaxanthin by Haematococcus pluvialis: taking the one-step system outdoors. Biotechnol Bioeng 102:651–657
Kumar K, Dasgupta CN, Das D (2014) Cell growth kinetics of Chlorella sorokiniana and nutritional values of its biomass. Bioresour Technol 167:358–366
Hejazi MA, Holwerda E, Wijffels RH (2004) Milking microalga Dunaliella salina for beta-carotene production in two-phase bioreactors. Biotechnol Bioeng 85:475–481
Huang TY, Lu WC, Chu IM (2012) A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour Technol 123:8–14
Cordero BF, Obraztsova I, Couso I, Leon R, Vargas MA, Rodriguez H (2011) Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar Drugs 9:1607–1624
Dineshkumar R, Subramanian G, Dash SK, Sen R (2016) Development of an optimal light-feeding strategy coupled with semi-continuous reactor operation for simultaneous improvement of microalgal photosynthetic efficiency, lutein production and CO2 sequestration. Biochem Eng J 113:47–56
Johnson EM, Kumar K, Das D (2014) Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour Technol 166:541–547
Sahu DK, Priyadarshani I, Rath B (2012) Cyanobacteria—as potential bio-fertilizer. CIBTech J Microbiol 1:20–26
Sholkamy EN, El-Komy H, Al-Arfaj AA, Abdel-Megeed A, Mostafa AA (2012) Potential role of Nostoc muscorum and Nostoc rivulare as biofertilizers for the enhancement of maize growth under different doses of N-fertilizer. Afr J Microbiol Res 6:7435–7448
Bocchi S, Malgioglio A (2010) Azolla-anabaena as a bio-fertilizer for rice paddy fields in the Po valley, a temperate rice area in northern Italy. Int J Agron 2010:1–5
Malliga P, Uma L, Subramanian G (1996) Lignolytic activity of the cyanobacterium Anabena azollae ML2 and the value of coir waste as a carrier for biofertilizer. Microbios 6:175–183
Silva PG, Silva HJ (2007) Effect of mineral nutrients on cell growth and self-flocculation of Tolypothrix tenuis for the production of a bio-fertilizer. Bioresour Technol 98:607–611
Dubey AK, Rai AK (2013) Application of algal biofertilizers (Aulosira Fertilissima Tenuis and Anabaena Doliolum Bhardawaja) for sustained paddy cultivation in Northern India. Israel J Plant Sci 43:41–51
Grzesik M, Romanowska-Duda Z, Kalaji HM (2017) Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 55:510–521
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Palabhanvi B, Muthusivaramapandian Muthurai M, Kumar V, Mukherjee M, Saumya Ahlawat S, Das D (2017) Continuous cultivation of lipid rich microalga Chlorella sp. FC2 IITG for improved biodiesel productivity via control variable optimization and substrate driven pH control. Bioresour Technol 224:481–489
Ghosh S, Roy S, Das D (2015) Improvement of biomass production by Chlorella sp. MJ 11/11 for use as a feedstock for biodiesel. Appl Biochem Biotechnol 175:3322–3335
Sharma A, Arya SK (2017) Hydrogen from algal biomass: a review of production process. Biotechnol Rep (Amst) 15:63–69
Basak N, Jana AK, Das D (2016) CFD modeling of hydrodynamics and optimization of photofermentative hydrogen production by Rhodopseudomonas palustris DSM 123 in annular photobioreactor. Internat J Hydrog Energy 41(18):7301–7317
Baltrėnas P, Misevičius A (2015) Biogas production experimental research using algae. J Environ Health Sci Eng 13:18. https://doi.org/10.1186/s40201-015-0169-z
Malik VS (2014) Editorial biofuel: the butanol perspective and algal biofuel. J Plant Biochem Biotechnol 23:337–338
Elmoraghy M, Farag IH (2012) Bio-jet fuel from microalgae: reducing water and energy requirements for algae growth. Internat J Eng Sci 1:22–30
Sathasivam R, Radhakrishnan R, Hashem A, AbdAllah EF (2019) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci 26:709–722
Lenihan-Geels G, Bishop KS, Ferguson LR (2013) Alternative sources of omega-3 fats: Can we find a sustainable substitute for fish? Nutrients 5:1301–1315
Martins DA, Custódio L, Barreira L, Pereira H, Ben-Hamadou R, Varela J, Abu-Salah KM (2013) Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs 11:2259–2281
Huang Y, Zhang D, Xue S, Wang M, Cong W (2016) The potential of microalgae lipids for edible oil production. Appl Biochem Biotechnol 180:438–451
Alam F, Date A, Rasjidia R, Mobin S, Moria H, Abdul Baqui A (2012) Biofuel from algae. Is it a viable alternative? Proc Eng 49:221–227
Ritchie RJ, Larkum AWD (2012) Modelling photosynthesis in shallow algal production ponds. Photosynthetica 50:481–500
Oswald WJ, Golueke CG (1960) Biological transformation of solar energy. Adv Appl Microbiol 11:223–242
Suh IS, Lee CG (2003) Photo-bioreactor engineering: design and performance. Biotechnol Bioproc Eng 8:313–321
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Gupta PL, Lee SM, Choi HJ (2015) A mini review: photobioreactors for large scale algal cultivation. World J Microbiol Biotechnol 31:1409–1417
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Molina Grima E, Acién Fernández FG, García Camacho F, Chisti Y (1999) Photobioreactors: light regime, mass transfer, and scale up. J Biotechnol 70:231–247
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol 57:287–293
Pereira H, Páramo J, Silva J, Marques A, Barros A, Maurício D, Santos T, Schulze P, Barros R, Gouveia L, Barreira L, Varela J (2018) Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: from an agar plate to 100-m3 industrial photobioreactors. Sci Rep 8:1–11
Rogers JN, Rosenberg JN, Guzman BJ, Oh VH, Mimbela LE, Ghassemi A, Betenbaugh MJ, Oyler GA, Marc D, Donohue MD (2014) A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res 4:76–88
Beal CM, Gerber LN, Sills DL, Huntley ME, Machesky SC, Walsh MJ, Tester JW, Archibald I, Granados J, Greene CH (2015) Algal biofuel production for fuels and feed in a 100-ha facility: a comprehensive techno-economic analysis and life cycle assessment. Algal Res 10:266–279
Singh SP, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sustain Energy Rev 38:172–179
Kumar K, Banerjee D, Das D (2014) Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana. Bioresour Technol 152:225–233
Richardson JW, Johnson MD, Zhang X, Zemke P, Chen W, Hu Q (2014) A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res 4:96–104
Decker EL, Reski R (2007) Current achievements in the production of complex biopharmaceuticals with moss bioreactors. Bioprocess Biosyst Eng 31:3–9
Camacho FG, Gomez AC, Fernandez FGA, Sevilla JF, Grima EM (1999) Use of concentric-tube airlift photobioreactors for microalgal outdoor mass cultures. Enzyme Microb Technol 24:164–172
Vega-Estrada J, Montes-Horcasitas MC, Domínguez-Bocanegra AR, Cañizares-Villanueva RO (2005) Haematococcus pluvialis cultivation in split-cylinder internal-loop airlift photo-bioreactor under aeration conditions avoiding cell damage. Appl Microbiol Biotechnol 68:31–35
Chiu SY, Tsai MT, Kao CY, Ong SC, Lin CS (2009) The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng Life Sci 9:254–260
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photo- bioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Xu L, Weathers PJ, Xiong XR, Liu CZ (2009) Microalgal bioreactors: challenges and opportunities. Eng Life Sci 9:178–189
Milner HW (1953) Rocking tray, algal culture from laboratory to pilot plant. Carnegie Inst Wash Publ 600:108–113
Ortega RD, Roux JC (1986) Production of Chlorella biomass in different types of flat bioreactors in temperate zones. Elsevier Biomass 10:141–156
Hu Q, Guterman H, Richmond A (1996) A flat inclined modular photo-bioreactor for outdoor mass cultivation of phototrophs. Biotechnol Bioeng 51:51–60
Wang B, Lan CQ, Horsman M (2012) Closed photobioreactors for production of microalgal biomasses. Biotechnol Adv 30:904–912
Hu Q, Faiman D, Richmond A (1998) Optimal orientation of enclosed reactors for growing photoautotrophic microorganisms outdoors. J Ferment Biotechnol 85:230–236
Tredici MR, Bassi N, Prussi M, Biondi N, Rodolfi LG, Chini Zittelli G, Sampietro G (2015) Energy balance of algal biomass production in a 1-ha green wall panel plant: how to produce algal biomass in a closed reactor achieving a high net energy ratio. Appl Energy 154:1103–1111
Chaumont D, Thepenier C, Gudin C (1988) Scaling up a tubular photoreactor for continuous culture of Porphyridium cruentum—from laboratory to pilot plant. Algal Biotechnology. Elsevier, London, pp 199–208
Molina E, Fernandez J, Acién FG, Chisti Y (2001) Tubular photo-bioreactor design for algal cultures. J Biotechnol 92:113–131
Morita M, Watanabe Y, Okawa T, Saiki H (2001) Photosynthetic productivity of conical helical tubular photo-bioreactors incorporating Chlorella sp. under various culture medium flow conditions. Biotechnol Bioeng 74:136–144
Morita M, Watanabe Y, Saiki H (2002) Photosynthetic productivity of conical helical tubular photobioreactor incorporating Chlorella sorokiniana under field conditions. Biotechnol Bioeng 77:155–162
Henrard AA, de Morais MG, Costa JAV (2011) Vertical tubular photo-bioreactor for semi-continuous culture of Cyanobium sp. Bioresour Technol 102:4897–4900
Travieso L, Hall D, Rao K, Benitez F, Sanchez E, Borja R (2001) A helical tubular photobioreactor producing Spirulina in a semi-continuous mode. Internat Biodeter Biodegrad 47:151–155
Ugwu CU, Ogbonna JC, Tanaka H (2005) Light/dark cyclic movement of algal culture (Synechocystis aquatilis) in outdoor inclined tubular photobioreactor equipped with static mixers for efficient production of biomass. Biotechnol Lett 27:75–78
De Vree JH, Rouke Bosma R, Janssen M, Barbosa MJ, Wijffels RH (2015) Comparison of four outdoor pilot-scale photobioreactors. Biotechnol Biofuels 8:215
Zhu H, Zhu C, Cheng L (2017) Plastic bag as horizontal photobioreactor on rocking platform driven by water power for culture of alkalihalophilic cyanobacterium. Bioresour Bioprocess 4:46
Tredici MR, Biondi N, Bassi N, Sampietro G (2016) Techno-economic analysis of micro-algal biomass production in a 1-ha green wall panel plant. Algal Res 19:253–263
Coelho RS, Vidotti ADS, Reis EM, Franco TT (2014) High cell density cultures of microalgae under fed-batch and continuous growth. Chem Eng Trans 38:313–318
Doucha J, Lívanský K (2012) Production of high-density Chlorella culture grown in fermenters. J Appl Phycol 24:35–43
Kaewpintong K, Shotipruk A, Powtongsook S, Pavasant P (2007) Photoautotrophic high-density cultivation of vegetative cells of Haematococcus pluvialis in airlift bioreactor. Bioresour Technol 98:288–295
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Roostaei J, Zhang Y, Kishore Gopalakrishnan K, Ochocki AJ (2018) Mixotrophic microalgae biofilm: a novel algae cultivation strategy for improved productivity and cost efficiency of biofuel feedstock production. Sci Rep 8:1–10
Zhang Q, Liu C, Li Y, Yu Z, Chen Z, Ye T, Wang X, Hu Z, Liu S, Xiao B, Jin S (2017) Cultivation of algal biofilm using different lignocellulosic materials as carriers. Biotechnol Biofuels 10:1–16
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Podola B, Li T, Melkonian M (2017) Porous substrate bioreactors: a paradigm shift in micro-algal biotechnology. Trends Biotechnol 35:121–132
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement
The enhancement of efficiency of photo-bioreactors should not be discouraged, and it is expected that improved designs would make them commercially feasible like solar cells.
Rights and permissions
About this article
Cite this article
Nigam, A., Sharma, A. Photo-bioreactors: Harnessing Solar Energy in Biological Way. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 723–732 (2020). https://doi.org/10.1007/s40011-019-01132-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-019-01132-7