Abstract
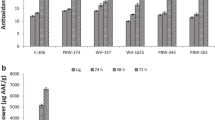
Germination of legumes results in some important biochemical and nutritional changes that may improve their nutritional status and be beneficial to human health. Present investigation reports the effect of germination time (3 and 6 days) and temperature (20 and 30 °C) on the phenolic constituents, bioactive compounds and antioxidant activity of germinating soybean genotypes varying in seed coat colour. Germination at 30 °C for 3 days resulted in highest total phenols, flavonols, tannins, saponins, ascorbic acid and tocopherols in most of the genotypes. Both germination time and temperature significantly influenced the antioxidant activity in germinated soybean flour as compared to raw seeds. 2,2-diphenyl, 1-picrylhydrazyl radical scavenging activity, total reducing power and nitric oxide radical scavenging activity were positively correlated with the total phenolic content (p < 0.05). Maximum contents of ascorbic acid, tocopherols and anthocyanins along with highest mean antioxidant activities were observed in germinating flour of black genotypes. Studies suggested that black coloured soybean seeds germinated at 30 °C for 3 days can be considered better from nutritional point of view than soybean with yellow, brown and green seed coat colour due to high antioxidants and low anti-nutrients.

Similar content being viewed by others
References
Haung X, Cai W, Xu B (2014) Kinetic changes of nutrients and antioxidant capacities of germinated soybean (Glycine max l.) and mung bean (Vigna radiata l.) with germination time. Food Chem 143:268–276
Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N (2008) Soy isoflavones exert antidiabetic and hypolipidemic effects through the par pathways in obese Zucker rats and murine raw 264.7 cells. J Nutr 133:1238–1243
Lee JH, Kang NS, Shin SO, Shin SH, Lim SG, Suh DY, Baek IY, Park KY, Ha TJ (2009) Characterization of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem 112(1):226–231
Rusydi MR, Azrina A (2012) Effect of germination on total phenolic, tannin and phytic acid contents in soybean and peanut. Food Res Int 19(2):673–677
Sharma S, Goyal R, Barwal S (2013) Domestic processing effects on physicochemical, nutritional and anti-nutritional attributes in soybean (Glycine max L. Merill). Food Res Int 20(6):3203–3209
Xu B, Chang SK (2008) Antioxidant capacity of seed coat, dehulled bean and whole black soybeans in relation to their distribution of total phenolic acids, anthocyanins and isoflavones. J Agric Food Chem 56(18):8365–8373
Xu B, Chang SK (2008) Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J Agric Food Chem 56:7165–7175
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica: the quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68
Balabaa SI, Zake AY, Elshamy AM (1974) Total flavonol and rutin content of the different organs of Sophora japonica L. J Assoc Off Anal Chem 57:752–755
Sadasivam S, Manickam A (1992) Phenolics. Biochemical methods for agricultural sciences. Wiley Eastern Ltd., New Delhi, pp 187–188
Fenwick DE, Oakenfull D (1983) Saponin content of food plants and some prepared foods. J Sci Food Agric 34:186–191
Kayden HJ, Chow CK, Bjomson LK (1973) Spectrophotometric method for determination of tocopherol in red blood cells. J Lipid Res 14(5):533–540
Okumara MA (1981) Specific method for the determination for total ascorbic acids in urine by α, α-bipyridyl method. Clin Chim Acta 115:393–403
Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y (1996) Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picryl hydrazyl radical. Free Radic Biol Med 21:895–902
Olabinri BM, Odedire OO, Olaleye MT, Adekunle AS, Ehigie LO, Olabinri PF (2010) In vitro evaluation of hydroxyl and nitric oxide radical scavenging activities of artemether. Res J Biol Sci 5(1):102–105
Halliwell B, Gutteridge JMC, Aruoma OI (1987) The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Lopez-Amoros ML, Hernandez T, Estrella I (2006) Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Comp Anal 19:277–283
Saharan K, Khetarpaul N, Bishnoi S (2002) Antinutrients and protein digestibility of Faba bean and Rice bean as affected by soaking, dehulling and germination. J Food Sci Technol 39:418–422
Shimelis EA, Rakshit SK (2007) Effect of processing on nutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa. Food Chem 103:161–172
Khandelwal S, Udipi SA, Ghugre P (2010) Polyphenols and tannins in Indian pulses: effect of soaking, germination and pressure cooking. Food Res Int 43:526–530
Kim JS, Kim JG, Kim WJ (2004) Changes in isoflavones and oligosaccharides of soybeans during germination. Korean J Food Sci Technol 2:294–298
Hallbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Plant Mol Biol 40:347–369
Yu O, Jung W, Shi J, Croes RA, Fader GM, McGonigle B (2000) Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. J Plant Physiol 124:781–794
Cevallos-Casals BA, Cisneros-Zevallos L (2010) Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem 119:1485–1490
Zhu D, Hettiarachchy NS, Horax R, Chen P (2005) Isoflavone cotents in germinated soybean seeds. Plant Foods Hum Nutr 60:147–151
Guajardo-Flores D, Serna-Saldivar SO, Gutierrez-Uribe JA (2013) Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.). Food Chem 141:1497–1503
Tarzia BG, Gharachorloo M, Baharinia M, Mortazavi SA (2012) The effect of germination on phenolic content and antioxidant activity of chickpea. Iran J Pharm Res 11(4):1137–1143
Yang MD, Chen J, Zhang XY, Zhang W (2010) Optimization of germination conditions for soybean. J Food Sci 31:97–101
Gujral HS, Angurala M, Sharma P, Singh J (2012) The Phenolic content and antioxidant activity of germinated and cooked pulses. Int J Food Prop 14:1366–1374
Acknowledgments
The authors wish to thank Dr. B S Gill, Senior Plant Breeder for providing soybean seeds. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miglani, H., Sharma, S. Impact of Germination Time and Temperature on Phenolics, Bioactive Compounds and Antioxidant Activity of Different Coloured Soybean. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 175–184 (2018). https://doi.org/10.1007/s40011-016-0744-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0744-9