Abstract
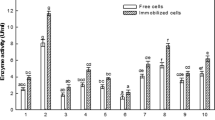
Asparaginase is a very important antineoplastic drug extensively used for the treatment of acute lymphoblastic leukemia and other tumor malignancies. But intrinsic glutaminase activity of this enzymatic drug is responsible for serious life threatening side effects. In this report, 154 bacterial isolates were isolated from rhizosphere of different plants and river water. All isolates were screened for glutaminase-free periplasmic asparaginase activity, and it was found that only four isolates (i.e., BO1, CO1, CO3 and GG1) lacked detectable glutaminase activity. Their measured asparaginase activities ranged from 0.1 to 0.37 IU/ml. These glutaminase-free asparaginase producing bacterial isolates were identified as Pseudomonas otitidis, Enterobacter cloacae, Ochrobactrum anthropi and Escherichia fergusonii, on the basis of morphological, cultural, biochemical characteristics and 16S rRNA gene sequencing. Crude enzyme extracted from these strains was screened for antitumor activity. Antitumor results showed that asparaginase obtained from P. otitidis and E. cloacae possesses potent antiproliferative effect on human leukemia (MOLT-4) and breast cancer (T47D and MDA-MB-231) cell lines, as compared to standard E. coli asparaginase preparation (Sigma), used in the present study. Based on these results, the asparaginase obtained from P. otitidis and E. cloacae could be used as a potent antiproliferative agent. However, more indepth studies are required for strengthening the current findings.


Similar content being viewed by others
References
Kang TS, Stevens RC (2009) Structural aspects of therapeutic enzymes to treat metabolic disorders. Hum Mutat 30:1591–1610
Verma N, Kumar K, Kaur G, Anand S (2007) L-Asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol 27:45–62
Asselin BL, Ryan D, Frantz CN et al (1989) In vitro and in vivo killing of acute lymphoblastic leukemia cells by l-asparaginase. Cancer Res 49:4363–4368
Lubkowski J, Palm GJ, Gilliland GL, Derst C, Rohm KH (1996) Crystal structure and amino acid sequence of Wolinella succinogenes l-asparaginase. Eur J Biochem 241:201–207
Gong SS, Basilico C (1990) A mammalian temperature-sensitive mutation affecting G1 progression results from a single amino acid substitution in asparagine synthetase. Nucleic Acids Res 18:3509–3513
Narta UK, Kanwar SS, Azmi W (2007) Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol 61:208–221
Geckil H, Gencer S, Ates B, Ozer U, Uckun M, Yilmaz I (2006) Effect of Vitreoscilla hemoglobin on production of a chemotherapeutic enzyme, l-asparaginase, by Pseudomonas aeruginosa. Biotechnol J 2:203–208
Kravchenko OV, Kislitsin YA, Popov AN, Nikonov SV, Kuranova IP (2008) Three dimensional structures of l-asparaginase from Erwinia carotovora complexed with aspartate and glutamate. Acta Crystallogr D 64:248–256
Kumar S, Dasu VV, Pakshirajan K (2011) Purification and characterization of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour Technol 102:2077–2082
Geckil H, Ates B, Gencer S, Uckun M, Yilmaz I (2005) Membrane permeabilization of gram-negative bacteria with a potassium phosphate/hexane aqueous phase system for the release of l-asparaginase: an enzyme used in cancer therapy. Process Biochem 40:573–579
Wriston JC Jr (1070) Asparaginase. Methods Enzymol XVII:732–742
Imada A, Igarasi S, Nakahama K, Isono M (1973) Asparaginase and glutaminase activities of microorganisms. J Gen Microbiol 76:85–99
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mac-Faddin FJ (1980) Biochemical tests for identification of medical bacteria. Williams and Wilkins, Baltimore
Kreig RN, Holt G (1984) Bergey’s manual of systematic bacteriology. William and Wilkins, Baltimore
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods 65:55–63
Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui C (2011) l-Asparaginase treatment in acute lymphoblastic leukemia. Cancer 15:238–249
Jakubas PB, Kulis MK, Giebel S et al (2008) Use of l-asparaginase in acute lymphoblastic leukemia: recommendations of the Polish Adult Leukemia Group. Pol Arch Med Wewn 118:664–669
Ohnuma T, Bergel F, Bray RC (1967) Enzymes in cancer: asparaginase from chicken liver. Biochem J 103:238–245
Broome JD (1965) Antilymphoma activity of l-asparaginase in vivo: clearance rates of enzyme preparation from guinea pig serum and yeast in relation to their effect on tumor growth. J Natl Cancer Inst 35:967–974
Mashbum LT, Wriston JC (1964) Tumor inhibitory effect of l-asparaginase from Escherichia coli. Arch Biochem Biophys 105:450–452
Yano S, Minato R, Thongsanit J, Tachiki T, Wakayama M (2008) Overexpression of type-I L-asparaginase of Bacillus subtilis in Escherichia coli, rapid purification and characterisation of recombinant type-I l-asparaginase. Ann Microbiol 58:711–716
Kim KW, Kamerud JQ, Livingston DM, Roon RJ (1988) Asparaginase II of Saccharomyces cerevisiae: characterization of the ASP3 gene. J Biol Chem 263:11948–11953
Reddy VVS, Jayaram HN, Sirsi M, Ramakrishnan T (1969) Inhibitory activity of l-asparaginase from Mycobacterium tuberculosis on Yoshida ascites sarcoma in rats. Arch Biochem Biophys 132:262–267
Cedar H, Schwartz JH (1968) Production of l-asparaginase II by Escherichia coli. J Bacteriol 96:2043–2048
Oza VP, Parmar PP, Kumar S, Subramanian RB (2010) Anticancer properties of highly purified l-asparaginase from Withania Somnifera L. against Acute Lymphoblastic Leukemia. Appl Biochem Biotechnol 160:1833–1840
Davidson L, Brear DR, Wingard P, Hawkins J, Kitto GB (1977) Purification and properties of an l-glutaminase-l-asparaginase from Pseudomonas acidovoranans. J Bacteriol 129:1379–1386
Acknowledgments
Authors are thankful to Head, Department of P.G. Studies and Research in Biological Science, Rani Durgavati University, Jabalpur for providing Laboratory facilities and Dr. Fayaz A. Malik, Scientist, Department of Pharmacology and Cancer Biology, Indian Institute of Integrative Medicine (IIIM-CSIR), Jammu, India, for providing Laboratory facilities for screening of antitumor activity of enzymes. Authors are also thankful to Madhya Pradesh Biotechnology Council, Bhopal, for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, A., Husain, I. Evaluation of Antitumor Activity of Glutaminase-Free Periplasmic Asparaginase from Indigenous Bacterial Isolates as Candidates for Cancer Therapy. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 87, 997–1004 (2017). https://doi.org/10.1007/s40011-015-0681-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0681-z