Abstract
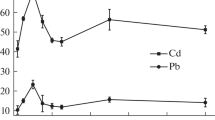
The present communication describes the development and application of black rice modified carbon paste electrode (BRMCPE) for the trace determination of Pb(II), Cd(II), Cu(II) and Zn(II) by square wave anodic stripping voltammetry. Electrochemical impedance spectra showed reduction of charge transfer resistance and higher electrocatalytic behavior of the sensor also supported by the cyclic voltammetry characterization of the electrode. The electrochemical parameters were optimized with reference to the amount of modifier, supporting electrolyte, accumulation time and accumulation potential. Mutual interference of the ions and the effect of surfactants on the metal ions were also investigated. Experimental analysis via BRMCPE under optimized conditions resulted in stripping responses with good linearity in the range 50–200, 400–1000, 250–700, 400–1000 μg/l and low limits of detection being 14.58, 154.83, 125.32, 134.04 μg/l respectively for Pb(II), Cd(II), Cu(II) and Zn(II).






Similar content being viewed by others
References
Torma F, Grun A, Bitter I, Toth K (2009) Calixarine/nafion-modified bismuth-film electrodes for adsorptive stripping voltammetric determination of lead. Electroanalysis 21(17–18):1961–1969
Jannata B, Sadeghib N, Oveisib MR, Behfarc A, Komeilizadehd H, Shafaatid A (2009) Simultaneous determination of lead, cadmium, copper and zinc in infant formula by anodic stripping voltammetry. Iran J Pharm Res 8(3):159–162
Gupta VK, Jain AK, Maheshwari G, Lang H, Ishtaiwi Z (2006) Copper (II)-selective potentiometric sensors based on porphyrins in PVC matrix. Sens Actuat B 117:99–106
Gupta VK, Singh AK, Mehtab S, Gupta B (2006) Cobalt(II)-selective PVC membrane based on a schiff base complex of N,N’-bis(salicylidene)-3,4-diaminotoluene. Anal Chim Acta 566:5–10
Gupta VK, Jain AK, Kumar P (2006) PVC-based membranes of n,n′-dibenzyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane as Pb(II)-selective sensor. Sens Actuat B 120:259–265
Gupta VK, Jain AK, Kumar P, Agarwal S, Maheshwari G (2006) Chromium(III)-selective sensor based on tri-o-thymotide in PVC matrix. Sens Actuat B 113:182–186
Jain AK, Gupta VK, Singh LP, Raisoni JR (2006) Comparative study of Pb2+ selective sensors based on derivatized tetrapyrazole and calix[4]arene receptors. Electrochim Acta 51:2547–2553
Gupta VK, Goyal RN, Sharma RA (2009) Comparative studies of neodymium (III)-selective PVC membrane sensors. Anal Chim Acta 647:66–71
Goyal RN, Gupta VK, Bachheti N, Sharma RA (2008) Electrochemical sensor for the determination of dopamine in presence of high concentration of ascorbic acid using a fullerene-C60 coated gold electrode. Electroanalysis 20:757–764
Goyal RN, Gupta VK, Bachheti N (2007) Fullerene-c60-modified electrode as a sensitive voltammetric sensor for detection of nandrolone—an anabolic steroid used in doping. Anal Chim Acta 597:82–89
Gupta VK, Singh AK, Khayat MA, Gupta B (2007) Neutral carriers based polymeric membrane electrodes for selective determination of mercury (II). Anal Chim Acta 590:81–90
Goyal RN, Gupta VK, Oyama M, Bachheti N (2007) Voltammetric determination of adenosine and guanosine using fullerene-C60-modified glassy carbon electrode. Talanta 71:1110–1117
Gupta VK, Singh AK, Gupta B (2007) Schiff bases as cadmium(II) selective ionophores in polymeric membrane electrodes. Anal Chim Acta 583(2):340–348
Goyal RN, Gupta VK, Sangal A, Bachheti N (2005) Voltammetric determination of uric acid at a fullerene-C60-modified glassy carbon electrode. Electroanalysis 17:2217–2223
Gupta VK, Chandra S, Lang H (2005) A highly selective mercury electrode based on a diamine donor ligand. Talanta 66:575–580
Gupta VK, Prasad R, Kumar A (2003) Preparation of ethambutol-copper(II) complex and fabrication of PVC based membrane potentiometric sensor for copper. Talanta 60:149–160
Gupta VK, Chandra S, Mangla R (2002) Dicyclohexano-18-crown-6 as active material in PVC matrix membrane for the fabrication of cadmium selective potentiometric sensor. Electrochim Acta 57:1579–1586
Gupta VK, Mangla R, Khurana U, Kumar P (1999) Determination of uranyl ions using poly(vinyl chloride) based 4-tert-butylcalix[6]arene membrane sensor. Electroanalysis 11(8):573–576
Goyal RN, Oyama M, Gupta VK, Singh SP, Sharma RA (2008) Sensors for 5-hydroxytryptamine and 5-hydroxyindole acetic acid based on nanomaterial modified electrodes. Sens Actuat B 134(2):816–821
Gupta VK, Jain S, Khurana U (1997) A PVC-based pentathia-15-crown-5 membrane potentiometric sensor for mercury(II). Electroanalysis 7(6):478–480
Gupta VK, Jain R, Radhapyari K, Jadon N, Agarwal S (2011) Voltammetric techniques for the assay of pharmaceuticals—a review. Anal Biochem 408:179–196
Gupta VK, Ganjali MR, Norouzi P, Khani H, Nayak A, Agarwal S (2011) Electrochemical analysis of some toxic metals by ion-selective electrodes. Crit Rev Anal Chem 41:282–313
Gupta VK, Nayak A, Agarwal S, Singhal B (2011) Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb Chem High Throughput Screen 14(4):284–302
Gupta VK, Singh LP, Singh R, Upadhyay N, Sethi B (2012) A novel copper (II) selective sensor based on dimethyl 4,4′ (o-phenylene) bis(3-thioallophanate) in PVC matrix. J Mol Liq 174:11–16
Gupta VK, Sethi B, Sharma RA, Agarwal S, Bharti A (2013) Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4]-arene as a cationic receptor. J Mol Liq 177:114–118
Mojica EE, Micor JRL (2007) Utilization of plant refuses as component of heavy metal ion sensors in water samples. J Appl Sci Environ Manage 11(3):69–74
Wang F, Liu J, JuWu Y, Gao Y, Huang X (2009) Anodic stripping voltammetric determination of mercury(II) in water using a 4-tert-butyl-1-(ethoxycarbonylmethoxy)thiacalix[4]arene modified glassy carbon electrode. J Chin Chem Soc 56:778–784
Rajawat DS, Satsangee SP (2011) Voltammetric determination of Pb(II) ions by carbon paste electrode modified with lemon grass powder. Res J Chem Environ 15(3):55–59
Serrano N, Martn N, Daz-Cruz JM, Arino C, Esteban M (2009) Bismuth film electrode in metal complexation studies: stripping analysis of the Pb(II)-, Cd(II)-, and Zn(II)-binding with phthalate. Electroanalysis 21(3–5):431–438
Berbel F, Cortes J, Dıaz-Cruz JM, Arino C, Esteban M (1998) Anodic stripping voltammetry of metal ions in mixtures of ligands. Electroanalysis 10(6):417–422
Cobelo-Garca A, Santos-Echeand J, Prego R, Nietob O (2005) Direct simultaneous determination of Cu, Ni and V in seawater using adsorptive cathodic stripping voltammetry with mixed ligands. Electroanalysis 17(10):906–911
Heitzmann M, Basaez L, Brovelli F, Bucher C, Limosin D, Pereira E, Rivas BL, Royal G, Aman E, Moutet J (2005) Voltammetric sensing of trace metals at a poly(pyrrole-malonic acid) film modified carbon electrode. Electroanalysis 7(21):1970–1976
Buica GO, Bucher C, Moutet J, Royal G, Saint-Aman E, Ungureanu EM (2009) Voltammetric sensing of mercury and copper cations at poly(EDTA-like) film modified electrode. Electroanalysis 21(1):77–86
Won M, Yoon J, Shim Y (2005) Determination of selenium with a poly(1,8-diamino-naphthalene)-modified electrode. Electroanalysis 17(21):1952–1958
Tamer U, Ertas N, Gndogdu Y (2008) A selective film based on poly(3-octylthiophene) doped with dihydroxyanthraquinone sulfonate. Electroanalysis 20(16):1805–1810
Tamer U, Oymak T, Ertas N (2007) Voltammetric determination of mercury(II) at poly(3-hexylthiophene) film electrode. Effect of halide ions. Electroanalysis 19(24):2565–2570
Chow E, Gooding JJ (2006) Peptide modified electrodes as electrochemical metal ion sensors. Electroanalysis 18(15):1437–1448
Kozan JVB, Silva RP, Serrano SHP, Lima AWO, Angnes L (2007) Biosensing hydrogen peroxide utilizing carbon paste electrodes containing peroxidases naturally immobilized on coconut (Cocusnucifera L.) fibers. Anal Chim Acta 591:200–207
Liawrungrath S, Purachat P, Oungpipat W, Dongduen C (2008) Sunflower leaves tissue-based bioelectrode with amperometric flow-injection system for glycolic acid determination in urine. Talanta 77:500–506
Fiol N, de la Torre F, Demeyere P, Florido A, Villaescusa I (2007) Vegetable waste based sensors for metal ion determination. Sens Actuat B 122:187–194
Park SD, Sam Y, Kim SJ, Chang HI (2008) Isolation of anthocyanin from black rice (Heugjinjubyeo) and screening of its antioxidant activities. Kor J Microbiol Biotechnol 36(1):55–60
Pyrzynska K, Pe Kal A (2011) Flavonoids as analytical reagents. Crit Rev Anal Chem 41(4):335–345
Xia F, Zhang X, Zhou CH, Sun A, Dong Y, Liu Z (2010) Simultaneous determination of copper, lead and cadmium at hexagonal mesoporous silica immobilized quercetin modified carbon paste electrode. J Autom Methods Manage Chem. doi:10.1155/2010/824197
Švancara I, Walcarius A, Kalcher K, Vytřas K (2009) Carbon paste electrodes in the new millennium. Central Eur J Chem 7(4):598–656
Rodriguez-Saona LE, Wrolstad RE (2001) Extraction, isolation and purification of anthocyanins. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. Wiley, New York, pp F1.1.1–F1.1.11
Choi SW, Kang WW, Osawa T (1994) Isolation and identification of anthocyanins pigments in black rice. Foods Biotechnol 3:131–136
Lee JH, Kang NS, Shin SO, Shin SH, Lim SG, Suh DY, Baek IY, Park KY, Ha TJ (2009) Characterization of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem 112:226–231
Rezaei B, Damiri S (2008) Multiwalled carbon nanotubes modified electrode as a sensor for adsorptive stripping voltammetric determination of hydrochlorothiazide. IEEE Sens J 8(9):1523–1529
Gardea-Torresdey J, Darnall D, Wang J (1988) Bioaccumulation and measurement of copper at an alga-modified carbon paste electrode. Anal Chem 60:72–76
Tiemann KJ, Gamez G, Parsons JG, Bess-Oberto L (2001) Environmental analysis of copper ions with a carbon paste electrode modified with alfalfa biomass. Symposia papers presented before the Division of Environmental Chemistry. Am Chem Soc 41(1):219–222
Mojica EE, Merca FE, Micor JRL (2002) Fiber of kapok (Ceiba Petandra) as component of a metal sensor for lead in water samples. Philipp J Crop Sci 27(2):37–42
Ouangpipat W, Lelasattarathkul T, Dongduen C, Liawruangrath S (2003) Bioaccumulation and determination of lead using treated-Pennisetum-modified carbon paste electrode. Talanta 61(4):455–464
Mojica ERE, Merca FE (2005) Anodic stripping voltammetric determination of mercury(II) using lectin-modified carbon paste electrode. J Appl Sci 5(8):1461–1465
Elvińa RO, Mojica ERE (2005) Orange peel essential oil as component of a metal sensor for lead(II) ion determination in aqueous solutions. J Appl Sci Environ Manag 9(2):23–27
Mojica ERE, Tocino AB, Micor JRL, Deocaris CC (2005) A feather-trode sensor for detecting lead ions. Philipp J Sci 134(1):51–56
Mojica EE, Micor JRL, Deocaris CC (2005) KaCCFEHM-trode example of a radiation synthesized metal sensor with a multi composite modifier. J Appl Sci Res 1(1):99–102
Mojica EE, Micor JRL, Gomez SP, Deocaris CC (2006) Lead detection using a pineapple bioelectrode. Philipp Agric Sci 89:134–140
Mojica EE, Vidal JM, Pelegrina AB, Micor JRL (2007) Voltammetric determination of lead(II) ions at carbon paste electrode modified with banana tissue. J Appl Sci 7(9):1286–1292
Mojico ERE, Santos JH, Micor JRL (2007) Determination of lead using a feather modified carbon paste electrode using anodic stripping voltammetry. World Appl Sci J 2(5):512–518
Acknowledgments
Financial support from Ministry of Human Resource and Development, India under NMEICT project is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devnani, H., Rajawat, D.S. & Satsangee, S.P. Black Rice Modified Carbon Paste Electrode for the Voltammetric Determination of Pb(II), Cd(II), Cu(II) and Zn(II). Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 84, 361–370 (2014). https://doi.org/10.1007/s40010-013-0112-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-013-0112-6