Abstract
Purpose
Despite having a wide range of therapeutic advantages, dexamethasone (DEXM)-free formulations have some negative side effects that manifest over time. Polymeric nanocapsules (PNCs) exhibit a core-shell structure that can encapsulate and control the release of drug products. Accordingly, the present study aimed to develop a new nanoparticulate system, PNCs, as drug nanocarriers of DEXM and to exemplify the difference in safety profile regarding the gastropathic and cardiopathic effects of DEXM PNCs versus free DEXM.
Methods
Dexamethasone-loaded alginate nanocapsules were prepared using the nanoprecipitation technique and evaluated for different parameters. In-vivo assessment of the safety profile of the DEXMs (free and PNCs) necessitated three animal groups: vehicle, free DEXM, and DEXM PNCs groups. Treatments with DEXM were administered intraperitoneally, once daily, for 7 days. Stomach and heart samples were investigated for tissue damage. Tissue insults were assessed via macroscopic, biochemical, histopathological, and immunohistochemical analyses.
Results
The selected PNCs exhibited a small particle size of 287 ± 7.5 nm, a zeta-potential of -21.06 ± 0.23 mV, an encapsulation efficiency of 91.53 ± 0.5%, and a prolonged release profile for up to 48 h as compared with a free drug. Gastric damage indicators showed more serious mucosal damage with free DEXM, hemorrhagic ulcers, and enhanced oxidative stress than the DEXM PNCs. Biomarkers of cardiac damage were significantly elevated with free DEXM and significantly lower in the DEXM PNCs group.
Conclusion
Dexamethasone was successfully encapsulated into polymeric nanocapsules of sodium alginate coating polymer. The developed alginate nanocapsules exhibited desirable parameters and a superior anticipated side effect profile regarding gastric and cardiac damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The words corticosteroid and corticoid are used synonymously to refer to the particular class of steroidal hormones produced by the adrenal cortex. Mineralocorticoids, glucocorticoids, and androgens are produced using cholesterol as a precursor material for synthesis (Schimmer et al. 2011). Dexamethasone (DEXM) is one of countless glucocorticoids frequently prescribed to treat various illnesses (Bandyopadhyay et al. 1999). Exogenous glucocorticoids such as DEXM are frontline anti-inflammatory and immunosuppressive drugs used to treat asthma and inflammatory disorders such as connective tissue diseases, ulcerative colitis, and rheumatoid arthritis (Yamaguchi et al. 2007). Meanwhile, in COVID-19 treatment, DEXM is stated as the world’s principal treatment to decrease the mortality hazard expressively (Horby et al. 2021) and is still the lone beneficial agent shown to be effective in severely diseased patients (Chen et al. 2021). The adverse effects associated with glucocorticoids frequently develop with chronic use, including hypertension (88%), osteoporosis (46%), myopathy (50%), hypercholesterolemia (30%), and cataracts (14%) (Covar et al. 2000).
Glucocorticoids are widely recognized to have the potential to produce gastropathy, which is considered to be brought on by an increase in pepsin and gastric acid production. Other studies suggest that glucocorticoids trigger the mast cells to degranulate and release histamine, which might explain why acid production is enhanced (Swamy et al. 2011). Nonetheless, glucocorticoid-induced gastropathy and gastric ulcers are still a topic of controversy. Narum and colleagues (2014) conducted a systematic review and meta-analysis to determine if corticosteroids were linked to an increased risk of gastrointestinal bleeding or perforation. The authors concluded that corticosteroids increased gastrointestinal bleeding and perforation risk. Moreover, Tseng et al. (2015) found a strong relationship between short-term glucocorticoid exposure and peptic ulcer hemorrhage. Prednisolone therapy for three months, according to Hsiang et al. (2010), did not raise the risk of developing peptic ulcer disease, while Butler et al. (2019) found that corticosteroid users had a low rate of gastrointestinal bleeding. In experiments, corticosteroids reduce the formation of stomach cytoprotective prostaglandins, as well as the generation of mucus and the release of bicarbonate, all of which weaken the gastrointestinal mucosal defenses (Guslandi 2013). In addition, steroids ruin both angiogenesis and epithelial healing mechanisms in experimental ulcers (Luo et al. 2004).
Due to its anti-inflammatory effects, DEXM showed promising results in improving cardiovascular outcomes in severely ill COVID-19 patients. These seem to be associated with a significant reduction in myocardial injury and a significant decrease in pulmonary embolism (Jirak et al. 2022). However, some detrimental effects on cardiovascular health were reported. According to de Salvi Guimarães et al. (2017), DEXM-treated rats showed several cardiovascular abnormalities, including elevated blood pressure, diastolic dysfunction, cardiac fibrosis, and cardiomyocyte apoptosis. These pathologic cardiac remodeling and diastolic dysfunction were associated with impaired calcium handling and calcineurin signaling pathway activation. Mohamed and colleagues (2022) concluded that a high dose of DEXM can induce cardiac injury in rats via increasing oxidative stress, profibrotic signals, and apoptosis. Hence, preparations that can overwhelm these negative adverse effects and improve the therapeutic potential of glucocorticoids in their target tissues are immensely desired.
To overcome such shortcomings of DEXM, an appropriate nanoparticulate efficient, biodegradable, and biocompatible delivery system must be developed. Nanoparticles (NPs) offer perfect drug delivery systems showing better therapeutic efficacy than free drug forms. Several DEXM nanoformulations have been developed, including the encapsulation of DEXM in poly (D, L-lactide-co-glycolide) (PLGA) NPs, (Pan et al. 2020) liposomes, and niosomes (Jia et al. 2018). In addition, different nanostructured lipid carrier systems of DEXM were previously investigated. Nanoencapsulation methods provide an efficient drug loading inside the nanocarriers, therefore reducing the systemic adverse effects caused by some drugs (Mo et al. 2018; Kiss et al. 2020; Kumari et al. 2021). Several studies have shown the improved therapeutic potential of NPs at the target sites (Wang et al. 2010; Fichter et al. 2013). However, minimal studies have investigated their potential for adverse effects in comparison with the conventional drug forms.
Because of their core-shell structure, PNCs have attracted more interest in drug delivery applications. They are defined as polymeric vesicular structures with nano-dimension size range (1–1000 nm) consisting of a liquid-solid core surrounded by a polymeric shell for a high encapsulation efficiency. The shell structure plays a critical role in developing polymeric nanocapsules that can encapsulate, protect, and control the release of drug products (Deng et al. 2020). The characteristics of polymers significantly affect the stability, encapsulation efficiency, and release profile of the nanocapsule as a drug delivery system. Biocompatible polymeric materials are considered suitable candidates for nanocapsules preparation (Klippstein et al. 2015). Alginate is an anionic biocompatible polymer produced by marine brown algae. It is interesting as a biopolymer to prepare nanocapsules because of its safety, biocompatibility, biodegradability, non-toxicity, water solubility, mucoadhesion, and film formation properties (Deng et al. 2020).
The short residence time of a small molecular weight drug (< 20 kDa) in the peritoneal cavity may lead to frequent or continuous dosing. Several efforts were made to prevent the premature clearance of a drug or NPs, including using a hydrogel or a viscous mucoadhesive polymer solution such as sodium alginate and chitosan. Mucoadhesive nanocarriers have the advantage of remaining in the peritoneal cavity for a long duration with adequate drug concentration compared to a free drug that rapidly reaches the blood (Fraguas-Sánchez et al. 2020).
Thus, the present study aimed to develop a novel nanoparticulate system, namely polymeric nanocapsules, to serve as drug carriers for DEXM. After that, the study aimed to examine the difference between the safety profiles of DEXM-loaded PNCs and free DEXM formulations in systemic use. The study was planned to show the gastropathic and cardiopathic effects of a fixed dose of systemic DEXM-loaded PNCs versus free DEXM in rats after a fixed duration of administration.
The developed nanocapsules in this study may offer a promising approach for suppressing the systemic side effects of DEXM. Sodium alginate was selected as the shell-coating polymer to develop DEXM polymeric nanocapsules. In-vitro characterizations of the prepared DEXM polymeric nanocapsules were carried out for particle size, morphology, zeta-potential, in-vitro drug release, and Fourier Transform Infrared (FT-IR) Spectroscopy. The in-vivo performance of the DEXM alginate nanocapsules was examined and compared against the pure DEXM.
Materials and methods
Chemicals
Dexamethasone was obtained from Amirya Pharmaceuticals. Tween®80, oleic acid, ethanol, and acetone were purchased from Adwic, El-Naser Chemical Co., Egypt. Sodium alginate, cetyltrimethylammonium bromide (CTAB), and Pluronic F-68 were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents were of analytical reagent grade.
Animals
The protocol of the current study was in line with the international and institutional guidelines for the use and care of experimental animals at the Faculty of Medicine, Assiut University. The animal study protocol was approved by the ethical committee of the Faculty of Medicine, Assiut University (IRB: 04-2023-300100). The U.K. guidelines for animal experiments (EU Directive 2010/63/EU) have been adhered to.
The study was conducted on eighteen adult male Wistar rats purchased from the animal house of the Faculty of Medicine, Assiut University. Their weight ranged between 200 and 250 g. Rats were housed in groups in clean, spacious cages under standard laboratory conditions, including a well-ventilated room at an appropriate temperature (25 ± 5 °C), maintained with good lighting. They were fed standard rodent food and had unrestricted access to water.
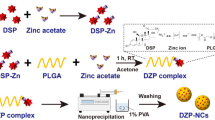
Preparation of DEXM loaded alginate nanocapsules (DEXM-Alg-NCs)
Dexamethasone-loaded alginate nanocapsules were prepared using the nanoprecipitation technique (Klippstein et al. 2015). Oleic acid and a cationic surfactant were dissolved in the internal organic phase. Dexamethasone (2 mg/0.5 ml) was added to the internal organic phase, composed of ethanol and acetone at a ratio of 1:1. The organic phase was added slowly drop-wise using a syringe to the aqueous phase containing sodium alginate polymer, calcium chloride (CaCl2) and a hydrophilic surfactant (Tween®80.) The formulated nanocapsules were kept for one hour under magnetic stirring at room temperature (Thermo Scientific and SA Co., China). The dispersion was then placed on a rotary evaporator (HAHNVAPOR HS- 2005 V, HAHNSHINE Scientific, Korea) under reduced pressure at 40 °C and 9000 rpm to ensure evaporation of organic solvents. The prepared nanocapsules were stored at 4–8 °C for further studies. The compositions of the prepared formulations are summarized in Table 1.
Characterization of DEXM alginate loaded nanocapsules
Evaluation of the particle size and zeta potential
The prepared nanocapsules were analyzed for their particle size and size distribution in terms of the average volume diameters and polydispersity index (P.D.I.) at 25 °C via dynamic light scattering (DLS) utilizing the particle size analyzer (Zetasizer Nano ZN, Malvern Panalytical Ltd, United Kingdom) at fixed angle of 173°. The zeta potential of the vesicles was estimated by laser Doppler anemometry using a Malvern Zetasizer ZS®. Samples were analyzed in triplicates.
Determination of Entrapment Efficiency (EE %)
Dexamethasone entrapment efficiency into alginate nanocapsules was estimated indirectly by quantifying the free drug in the aqueous supernatant using a UV spectrophotometer (LISCO GmbH Bargteheide, Germany) at 242 nm. The unentrapped drug was separated from drug-loaded nanocapsules by centrifugation at 4 °C, 18,000 rpm, for 60 min., using a bench-top refrigerated centrifuge (Centurion Scientific Ltd, Sussex, and UK). The developed nanocapsules were reconstituted in distilled water and washed twice using the same procedures. The EE % was calculated according to the following euation:
Morphology
The surface morphology of prepared DEXM-loaded nanocapsules was visualized using scanning electron microscopy (Jeol, JSM-5200, and Japan). The sample was prepared by placing a droplet onto an aluminum specimen stub, dried overnight, and sputter-coated with gold before imaging.
In-vitro drug release studies
The in-vitro release study of DEXM from DEXM-loaded nanocapsules and free DEXM suspension was performed using the dialysis membrane diffusion method. Phosphate buffer saline (PBS) pH 7.4 was selected as the release medium. 1 ml of the nanocapsule suspension and free drug solution was put over a previously soaked cellulose membrane (Spec-tro/Pore membranes, molecular weight cut-off 12–14 kDa) fitted at the lower end of a glass cylinder. The glass cylinder was then dipped in a beaker containing (100 ml) PBS systems at 37 ± 0.5 °C and stirred at 100 rpm using a thermostatically controlled water bath (Shaking bath, DAIHAN Scientific Co., Seoul, South Korea). Samples (5 mL) were collected at the pre-determined time for 24 h and substituted with a freshly prepared buffer medium to maintain sink conditions during the release studies. The drug content was measured using a UV spectrophotometer at 242 nm. The In-vitro release experiment was repeated in triplicates. Different mathematical models (zero-order, First-order, Higuchi diffusion, and Korsmeyer-Peppas) were applied to measure the kinetics and mechanism of drug release from the prepared PNC formulation.
Fourier Transform Infrared (FT-IR) spectroscopy
Infrared spectra of DEXM, oleic acid, CTAB, Tween 80, and the selected nanocapsules formulation were evaluated using a Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA). All samples were mixed with spectroscopic grade potassium bromide (KBr) and compressed into disks using a hydraulic press (15,000 Ib). Samples were scanned from 4000 to 400 cm-1.
Stability studies
The stability of the DEXM-Alg-NCs optimum formulation was evaluated by storing them at 4 °C and 25 °C in a sealed 20 mL glass vial. The size, PDI, and encapsulation efficiency values were recorded at predefined time intervals (fresh preparation and 4 weeks after formulation and storage). The appearance of dispersion was also monitored.
Drug administration and study design
Dexamethasone sodium phosphate (8 mg/2 ml) ampoules were obtained from Amirya Company for pharmaceutical industries in Egypt. Three animal groups were organized, with six rats (n = 6) allocated in each group: vehicle group, free DEXM group, and DEXM loaded PNCs group. Dexamethasone treatments (10 mg/kg) were given by intra-peritoneal route once daily for 7 days. This DEXM dose was previously reported to induce cardiac injury, according to Mohamed et al. (2022). Dexamethasone induced gastric mucosal damage in rats at 4 mg/kg doses for 4 days (El Zahaby et al. 2017; El-Lekawy et al. 2019). Animals were weighed before and 24 h after completion of treatment. Blood glucose levels were measured using a glucometer (FreeStyle Freedom® Lite) before the start of treatment and 24 h after the end of treatment.
Measurement of blood glucose level
Twenty-four hours after treatment termination, blood glucose was estimated using the glucometer (FreeStyle Freedom® Lite glucometer, TheraSense, Inc., USA). The tail incision method was used in blood sampling. One drop of blood from the tail vein was delicately applied to the test area of the glucometer, and blood glucose levels were recorded immediately in mg/dL.
Determination of body weight
Before sacrificing, the animals were weighed. The body weights were compared with those of the vehicle group and between treatment groups.
Blood sample collection, stomach, and heart isolation
Blood samples were humanely collected from the retro-orbital venous sinuses at the end of the treatment period for serum separation. The instructions of the National Center for the replacement, refinement, and reduction of animals in research (https://nc3rs.org.uk/rat-retro-orbital-non-surgicalterminal) were carefully followed in blood collection. Animals were sacrificed by cervical dislocation under diethyl ether anesthesia.
The stomachs and hearts were carefully dissected out. The stomachs were opened by the greater curvature and washed with normal saline for macroscopic examination of the gastric mucosa. Stomach and heart samples were cut into two parts; one was preserved in 10% formalin for pathology and immunohistochemistry, while the other was homogenized in normal saline (10% w/v) for biochemical evaluations in the supernatants.
Evaluation of gastric mucosal damage
Determination of oxidative stress markers: malondialdehyde (MDA), reduced glutathione (GSH)
Gastric MDA concentrations were measured spectrophotometrically using commercial kits purchased from Biodiagnostic, Giza, Egypt, Catalog Number: MD 25 28. The assay principle depends on the reaction of thiobarbituric acid (TBA) with MDA in an acidic medium at 95 °C for 30 min to form a thiobarbituric acid reactive product. The absorbance of the resultant pink product was measured at 534 nm, and the concentration was calculated and expressed as nmol/g tissue.
Gastric GSH concentrations were measured spectrophotometrically using commercial kits purchased from Biodiagnostic, Giza, Egypt, Catalog Number: GR 25 10. The assay principle depends on reducing 5,5` dithiobis (2- nitrobenzoic acid) (DTNB) with GSH to produce a yellow compound. The absorbance of the resultant product was measured at 405 nm, and the concentration was calculated and expressed as mmol/g tissue.
Gross examination
After euthanasia and sample collection, the stomach of the rats was removed and opened along the greater curvature to evaluate the degree of damage and ulceration. Different parts of the gastric mucosa were grossly inspected for the presence of various lesions to record their numbers and severity. The grade of lesions for each rat was scored according to the following ulcer score (MacAllister et al. 1997): 0 = no lesions, 1= (1– 2) superficial localized lesions, 2= (3–5) deep localized lesions, 3= (6–10) Multiple lesions, 4 = > 10 or deep hyperemic and/or darkened diffuse lesions.
The ulcer index for each group of rats was calculated agreeing with the following equation (Swarnakar et al. 2005).
Histopathological examination
Sections of the stomach flattened on a piece of cork with the mucosal surface up, containing lesions accompanied by sections of the heart muscle, were prepared. Stomach and heart samples were fixed in a 10% neutral buffered formalin solution for 24 h. After fixation, all samples were regularly processed for conservative histopathological examination. Five-micron sections were cut and stained with hematoxylin and eosin (H&E) stain (Bancroft et al. 2008) for histopathological examination by light microscopy (CX31; Olympus, Tokyo, Japan) and photographed using a digital camera (Toupview, LCMos10000KPA, china) in the Photomicrograph Lab. of Pathology, Clinical Pathology Department, Faculty of Veterinary Medicine, Assiut University (Suvarna et al. 2018). The histopathological findings for each group were presented in a tabulated form to demonstrate the type of lesion, severity, and percentage of animals.
Morphometric analysis
The H&E-stained stomach slides in all examined rats were subjected to morphometric analysis to measure the length of the gastric mucosa. The images were obtained and measured digitally using an Axiostar plus microscope (Carl Zeiss, Thornwood, NY, USA) interfaced with an Axiostar plus digital camera and Axiovision 4.1 software (Carl Zeiss). For each rat, multiple gastric sections were examined with a 10x objective lens, photographs were taken, and then 3 fields were randomly selected for analysis.
Evaluation of cardiopathic effects
Determination of cardiac creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH)
According to the test kit’s procedure, the creatine kinase-MB and lactate dehydrogenase levels were quantitatively determined by spectrophotometry using cardiac tissue homogenates.
Determination of cardiac superoxide dismutase (SOD) and malondialdehyde (MDA)
Cardiac SOD activity (Catalog No.: SD 25 20) and MDA (Catalog No.: MD 25 28) concentrations were measured spectrophotometrically using commercial kits purchased from Biodiagnostic, Giza, Egypt, following the enclosed instructions.
Histopathological examination
Sections of the heart muscle were prepared and fixed in a 10% neutral buffered formalin solution for 24 h. After fixation, all samples were regularly processed for conservative histopathological examination. Five-micron sections were cut and stained with H&E stain (Bancroft et al. 2008) for histopathological examination by light microscopy (CX31; Olympus, Tokyo, Japan) and photographed using a digital camera (Toupview, LCMos10000KPA, china) in the Photomicrograph Lab. of Pathology, Clinical Pathology Department, Faculty of Veterinary Medicine, Assiut University (Suvarna et al. 2018).
Immunohistochemistry of Caspase-3
All groups’ heart muscle paraffin slices were utilized to identify caspase-3 (A11953) using immunohistochemistry at a dilution of 1:100. The tissue sections were deparaffinized and rehydrated in a descending order of alcohols. The tissue sections were 3 mm thick. Utilizing a microwave and citrate buffer (pH 6), heat-induced antigen retrieval was carried out for 20 min using the method recommended by the antibody manufacturer. The endogenous peroxidase activities were neutralized with 3% hydrogen peroxide (H2O2). Afterward, tissues were incubated with a primary antibody for caspase-3 (A11953) diluted in PBS overnight at 4 °C in a humidified environment. The primary antibodies were detected in all experimental groups. Econo Tek biotinylated anti-polyvalent was applied and incubated for 30 min. Then, the tissues were rinsed four times for 5 min each with PBS, and the sections were incubated in Econo Tek HRP Conjugate for 30 min at room temperature. A mixture of DAB chromogen was visualized in the sections, and the DAB substrate was then incubated for 10 min. Sections were hydrated, then counterstained with hematoxylin, dehydrated, and mounted. Positive immunoreactions looked at the brown coloration. For the negative control, sections were incubated in a buffer without primary antibody.
Immunohistochemical staining for caspase-3 was scored by visual assessment of ten ×40 fields as follows: (1) 0–30% of cardiac muscle immunopositive, (2) 31–70% of cardiac muscle immunopositive, and (3) > 71% of cardiac muscle immunopositive. (Ummanni et al. 2010).
Statistical analysis
All findings were reported as means ± standard deviations (SD) or standard error (SE). One-way analysis of variance with Tukey Kramer repeated measures or Student’s t-test (GraphPad Prism 8.0, GraphPad, San Diego, CA) was employed to determine the statistically significant differences between the different groups. The significance was defined as p < 0.05.
Results
Characterization of DEXM-Alg-NCs
Dexamethasone alginate nanocapsules were successfully prepared using the nanoprecipitation method (Fig. 1). According to the preliminary studies, the concentration of oleic acid was fixed at 2.5% w/v from the final volume of nanocapsules dispersion because of the higher EE % obtained at this concentration. Sodium alginate was selected as a coating polymer according to previous preformulation trials to produce dexamethasone nanoformulation with acceptable appearance and colloidal stability. Also, sodium alginate (NaAlg) concentration was fixed at 0.05% w/v because of the significant increase in particle size and P.D.I was found to have a further increase in NaAlg concentration, possibly due to the resulting much higher viscosity of the aqueous phase. The appropriate appearance of the developed polymeric nanocapsule was observed using the concentrations mentioned above of NaAlg and oleic acid (Fig. 2A).
Table 1 shows the particle size, P.D.I., zeta potential, and encapsulation efficiency percentage of different DEXM Alg-PNC. It was noticed that the average particle size changed significantly (p < 0.05) between different formulations and ranged from 287 to 370 nm, with low P.D.I., which ranged from 0.22 to 0.5. The particles showed high negative zeta potential ranging from − 10 to − 21mV. DEXM-PNC5 that contains Tween®80 (0.03 g) CTAB (5 mg) showed a significant decrease (p < 0.05) in the particle size (287 ± 7.5 nm) as compared with other formulations. The surface morphology of the developed DEXM polymeric nanocapsules (DEXM-PNC5) was studied using scanning electron microscopy (Jirak et al. 2022) (Fig. 2B). The prepared nanocapsules showed homogenous monodispersed spherical-shaped particles with smooth surfaces. The observed SEM particle size matches that determined by the particle size analyzer.
Also, it was noticed that the presence of Pluronic F-68 resulted in a highly significant increase (p < 0.001) in particle size, and precipitation of nanocapsules was observed as compared with Tween®80 (Table 1). The effect of CTAB and Tween®80 different amounts on the different characterization parameters was studied, and the results revealed that the optimum amounts that produced desirable alginate nanocapsules with smaller particle size, P.D.I., and high negative value zeta-potential was 5 mg and 0.03 g, respectively.
The effect of Tween®80 amounts as a hydrophilic surfactant on the encapsulation of DEXM into alginate nanocapsules was exhibited in Table 1; the entrapment efficiency of DEXM was significantly increased (p < 0.05) by increasing Tween®80 amount as detected in PNC5 and PNC6 (91.53 ± 0.5 and 80.66 ± 1.15%, respectively) due to the hydrophilic and higher solubilizing effects of Tween®80.
According to the previous findings, PNC5 was selected for further studies, as it exhibited the highest value of EE % (91.53 ± 0.5%), the smallest size (287 ± 7.5 nm) with low P.D.I. (0.22), and the highest value of negative zeta-potential (-21.06 ± 0.23 mV).
(A) Visual appearance of DEXM-loaded NaAlg nanocapsules (DEXM-Alg-NC5 (PNC5). (B) Morphology of selected (DEXM-Alg-NC5) using a scanning electron microscope. (C) Cumulative in-vitro release profiles of DEXM from alginate nanocapsules DEXM-Alg-NC5 and from DEXM suspension in phosphate buffer saline (PHS, pH 7.4) at 37 °C. Data are expressed as mean ± SD (n = 3). (D) FT-IR spectra of (A) DEXM, (B) Tween 80, (C) CTAB, (D) NaAlg, (E) Oleic acid and (F) DEXM-Alg-NC5. DEXM: Dexamethasone, CTAB: cetyltrimethylammonium bromide, NaAlg: sodium alginate, DEXM-Alg-NC5: selected formulation of DEXM loaded polymeric nanocapsules containing sodium alginate as coat (0.05%), oleic acid (2.5%), CTAB (5 mg) and Tween 80 (0.03 g)
In-vitro release of DEXM from alginate nanocapsules
In-vitro release studies of selected formulation (PNC5) were carried out in PBS at pH 7.4 and 37 °C. The release profile of DEXM from the selected nanocapsules formulation (PNC5) was compared with that of DEXM suspension (Fig. 2C). As seen in the figure, DEXM-Alg-NC demonstrated a prolonged release profile for up to 48 h as compared with that of free drug suspension. DEXM-Alg-NC showed an initial drug release of 12.34% in the initial 2 h, followed by a sustained drug release (80.3%) over 48 h. In contrast, free DEXM suspension exhibited a complete drug release (99%) after 6 h; the released amount from DEXM-PNC was significantly different from DEXM suspension (p < 0.001). These findings are consistent with previous works investigating the possibility of oil-core nanocapsules being considered a controlled-release drug delivery system (Hatahet et al. 2017; El-Gogary et al. 2020).
The release data was analyzed using zero-order, first-order, Higuchi, and Korsmeyer– Peppas models to determine the best mechanism for drug release. The release rate constant (k) and correlation coefficient (R2) calculated by different mathematical models are presented in Table 2. The most appropriate model was determined based on the highest correlation coefficient (R2) value. The highest R2 value for the selected DEXM-Alg-NC5 formulation was best fitted to the Higuchi model, confirming that DEXM release is diffusion-controlled. The calculated n value was 0.75, corresponding to anomalous non-Fickian transport; this means that the mechanism of drug release is governed by polymer matrix erosion and diffusion. Our finding follows previous studies that explain the similar release patterns of nanocapsules (Marchiori et al. 2010; Torge et al. 2017).
Fourier Transform Infrared (FTIR) spectroscopy
The FTIR spectrum of DEXM, Tween®80, CTAB, NaAlg, oleic acid, and DEXM-Alg-NC are presented in Fig. 2D. The spectrum of DEXM displayed absorption peaks at 3390 and 3500 cm-1, which are the main characteristic beaks of DEXM. Moreover, the spectrum shows an absorption band at 1715 c, which matches the previously reported work (Yu et al. 2020). In the spectrum of DEXM-Alg-NC5, the characteristic beaks of DEXM disappeared. Further, the physical interaction in alginate nanocapsule formation resulted in the smoothing of the absorption beaks. No new beaks were observed in the nanocapsules spectrum, indicating no interactions between the medication and the preparation ingredients.
Stability studies
The stability of the selected formulation was evaluated at different time intervals for one month at two different temperatures (4 °C and 25 °C). As illustrated in Fig. 3, the selected formulation exhibited an insignificant decrease (p > 0.05) in its particle size and P.D.I. upon storage during the evaluated periods (Fig. 3 A&B). These results suggest that no particle aggregation was produced. Regarding the DEXM encapsulation, it was noticed that the formulation showed a non-significant change in EE % during storage at two temperatures, and no drug degradation was detected (Fig. 3 C). These observations indicate that DEXM encapsulated into the polymeric core remained stable for one month of storage.
Blood glucose levels
The effect of free DEXM and DEXM PNCs on the blood glucose levels in treated rats at the end of treatment was studied. A significant (p < 0.0001) reduction in blood glucose levels was observed in the free DEXM-treated and DEXM PNCs-treated rats compared to the vehicle group. A significant difference was further detected between the DEXM-treated groups (Table 3).
Body weight
The free and polymeric DEXM nanocapsules significantly (p < 0.0001) reduced rats’ body weight as measured at the end of treatment versus the vehicle group. The free DEXM reduced body weights by 28%, while the polymeric DEXM nanocapsules reduced it by only 14%. A significant (p < 0.0001) difference was further detected between the treatment groups (Table 3).
Effects on gastric mucosal damage
Gastric MDA and GSH concentrations
Treatment with free and polymeric DEXM nanocapsules significantly (p < 0.0001) elevated the gastric MDA concentrations in comparison with the vehicle group (429.5–204%, respectively). A significant difference (p < 0.0001) was further noticed between the free and polymeric DEXM nanocapsules treated groups. The concentration of MDA in the gastric tissues of the polymeric DEXM nanocapsules-treated rats was significantly lower than that in the free DEXM-treated rats (Table 3).
The gastric GSH concentrations were significantly (p < 0.0001) reduced in both groups treated with free and polymeric DEXM nanocapsules in comparison with the vehicle group (26–16%, respectively). A significant difference (p < 0.0001) was observed between the free and polymeric DEXM nanocapsules treated groups. The concentration of GSH in the gastric tissues of the polymeric DEXM nanocapsules-treated rats was significantly higher than that in the free DEXM-treated rats (Table 3).
Gross examination
Gross inspection of the stomach of experimental animals in different groups involved the fore-stomach and glandular stomach as exhibited (Fig. 4). The mucosal surface of the glandular part is divided into the fundus with reddish mucosa and the pylorus as a relatively whitish mucosa; however, the fore-stomach showed typical whitish coloration (Fig. 4A). Hemorrhagic ulcers with whitish necrotic foci were observed in the glandular part of the stomach of the free DEXM-treated group (Fig. 4B, C&D). The stomach of the DEXM PNCs-treated group showed normal gastric mucosa (Fig. 4E) with a small number of hemorrhagic ulcers in some sections (Fig. 4F). The ulcer score and index of all gross lesions observed are presented in Table 4.
Gross inspection of the stomach; (A) control rats showing typical gastric mucosa; (B, C&D) free DEXM-treated group showing hemorrhagic ulcers (arrows) and necrotic foci (notched arrows). (E) DEXM PNCs-treated group showing no injury of gastric mucosa. (F) DEXM PNCs-treated group showing small number of hemorrhagic ulcers (arrows) in gastric mucosa
Histopathological examination
The H&E-stained stomach sections of the vehicle rats revealed the normal histological features of the gastric mucosa (Fig. 5A). The gastric epithelium-lined mucosa showed normal construction with gastric pits (Fig. 5B). Microscopic examination of stomach sections from the free DEXM-treated group at different parts of the glandular gastric mucosa, frequently in the fundic and pyloric regions of the stomach, exhibited mild erosive and hemorrhagic gastric changes in 4 rats (Fig. 5C, D&E and F). Necrosis and desquamation of the lining epithelium were observed in different parts of gastric mucosa, associated with tissue debris (Fig. 5C). These changes were accompanied by edema in deep layers of gastric mucosa between gastric glands (Fig. 5D). Angiopathic lesions were observed in different gastric layers characterized by interstitial hemorrhage between gastric glands (Fig. 5E). Mucosal hyperemia was also observed in the lamina propria (Fig. 5F).
However, the DEXM PNCs-treated group exhibited very mild gastric changes. These changes were characterized by slight gastric epithelium desquamation and mucosal hyperemia (Fig. 5G). Congestion was also observed in other gastric layers, such as the muscular layer (Fig. 5H). The incidence of histopathological findings of the stomach in the experimental groups is summarized in Table 5.
Morphometric analysis
Data from the morphometric study of the stomach in all experimental groups supported the findings from tissue sections stained with H&E which were further displayed (Fig. 5 I). In various microscopic areas, the studied rats from the free DEXM-treated group displayed a significant decrease in the thickness of stomach mucosa compared to the vehicle group. Rats in the free DEXM-treated group demonstrated that the mean mucosal length was (732.10 ± 108.83) significantly lower than that of the vehicle group (1014.33 ± 215.03). The difference in stomach mucosa thickness between the DEXM PNCs-treated and vehicle groups was also significant. The mucosal thickness significantly increased in the DEXM PNCs-treated group compared to the free DEXM-treated group. A significant difference between the two groups indicated the milder injurious effect of DEXM PNCs. The thickness of mucosa significantly increased in DEXM PNCs-treated group when compared with free DEXM-treated group, and significant difference was found between the two groups indicating the protective effect of DEXM PNCs.
Histopathological examination of gastric mucosa, (A) vehicle group showing typical gastric mucosa, bar = 100. (B) Vehicle group showing the normal gastric mucosa lined with gastric epithelium, bar = 50. (C) Free DEXM-treated group showing erosive changes characterized by necrosis and desquamation of the lining epithelium (arrow), tissue debris in the gastric lumen (star), bar = 100. (D) Free DEXM-treated group showing edema in the deep layer of gastric mucosa between gastric glandes (arrow), bar = 20. (E) Free DEXM-treated group showing interstitial hemorrhage between gastric glands (arrows), bar = 20. (F) Free DEXM-treated group showing hyperemia in lamina propria (arrows), bar = 20. (G) DEXM PNCs-treated group showing slight desquamation of gastric epithelium (notched arrow) slight mucosal hyperemia (arrow), bar = 100. (H) Free DEXM-treated group showing congestion (arrow) in the muscular layer, bar = 100. H&E. (I) The mucosal thickness in different experimental groups. Values are expressed as means ± SE (n = 6). The p-value < 0.05 indicates significance. Free DEXM; free dexamethasone, DEXM PNCs; DEXM polymeric nanocapsules
Cardiopathic effects
Cardiac CK-MB and LDH
Biomarkers of cardiac damage, including CK-MB and LDH, were determined to evaluate the detrimental effects of free and polymeric DEXM nanocapsules. Treatment with free and polymeric DEXM nanocapsules significantly (p < 0.05) elevated the levels of CK-MB and LDH as compared with the vehicle group. A significant difference (p < 0.01) was further noticed between the free and polymeric DEXM nanocapsules treated groups (Table 6).
Cardiac SOD and MDA
Oxidative stress plays a critical role in the progression of cardiotoxicity, and SOD is an important anti-oxidative enzyme. Treatment with free and polymeric DEXM nanocapsules up-regulated SOD activity and amplified MDA concentration, suggesting the existence of oxidative damage within the cardiac tissues (Table 6). The concentration of MDA in the cardiac tissues of the free DEXM-treated rats was significantly higher compared to the vehicle group. Treatment with polymeric DEXM nanocapsules significantly (p < 0.01) lowered cardiac MDA concentration versus the free DEXM group.
A significant (p < 0.05) increase in SOD activity was observed in the cardiac tissues of free DEXM-treated rats compared to the vehicle group. A significant difference (p < 0.01) was observed between the free and polymeric DEXM nanocapsules-treated groups. The polymeric DEXM nanocapsules significantly (p < 0.01) lowered the increments in SOD activity compared to the free DEXM.
Cardiac histopathology
Microscopic examination of cardiac muscle in all groups showed mild variation in treated groups. The heart muscle of the vehicle group showed normal architecture (Fig. 6 A). At the same time, the histological sections of the myocardial muscle of the free DEXM-treated group revealed focal areas of cytoplasmic vacuoles in the myocardial fibers with myofibrillar loss (Fig. 6 B). The histopathological lesions of heart muscle were mild in the polymeric DEXM nanocapsules-treated groups. The loss of cellular constituents of the myocardial cells appeared in a few heart muscles of the polymeric DEXM nanocapsules-treated groups (Fig. 6 C).
Immunohistochemical expression of Caspase-3 in heart muscle
Expression of caspase-3 is a good apoptotic marker in heart tissue. No positive cells were detected in the vehicle group (Fig. 7 A&B). Most cardiac cells in the Free DEXM-administrated group strongly expressed caspase-3 (Fig. 7 C&D). A moderate positive reaction of caspase-3 was observed in the DEXM polymeric nanocapsules-treated group (Fig. 7 E&F). The statistical analysis of the immunohistochemical expression of caspase-3 was presented in the (Fig. 8).
Histopathological examination of cardiac muscle, (A) heart muscle of control rats showing normal architecture, bar = 20. (B) Free DEXM group showing focal areas of cytoplasmic vacuoles in myocardial fibers with myofibrillar loss (star), bar = 20. (C) DEXM polymeric nanocapsules group showing mild loss of cellular constituents of the myocardial cells in few heart muscle (star), bar = 20
Immunohistochemical staining for caspase-3 in heart sections, (A) vehicle group showing negative reaction, bar = 100. (B) Higher magnification of the vehicle group, bar = 20. (C) Free DEXM group showing severe positive reaction, bar = 100. (D) Higher magnification of free DEXM administrated group, bar = 20. (E) The DEXM polymeric nanocapsules-treated group showing moderate positive reaction, bar = 100. (F) Higher magnification of the DEXM polymeric nanocapsules-treated group, bar = 20
Discussion
According to a literature search, evaluating the negative effects of nano-drug formulations as nanocapsules was not effectively considered in the experimental trials. Hence, in this work, we attempted to create a novel nanoparticulate system, polymeric nanocapsules, as drug nanocarriers of DEXM and to highlight the safety profile of the DEXM polymeric nanocapsules and compare it to the free DEXM formulations during systemic usage. DEXM is a highly effective medication for treating various inflammatory conditions. The long-term treatment of chronic inflammatory illnesses, however, may have a variety of adverse consequences. These adverse effects are the primary drawbacks of long-term DEXM usage. DEXM’s anti-inflammatory effectiveness would be increased by being enclosed in nanosized alginate nanocapsules, which might also lessen the adverse effects of long-term DEXM use.
Many hydrophobic medications have been delivered via polymeric nanocapsules, which have been the subject of substantial investigation. The combination of the oil core and polymer shell was attributed to this. It has been demonstrated that the oil core is a good medium for encapsulating large volumes of lipophilic medications, enabling their administration and delivery into various tissues. Moreover, the coated polymer shell acted as a barrier, enhancing the stability of the nanoformulation and providing the encapsulated medication with prolonged release behavior (El-Sheridy et al. 2019).
For the first time, oil-core polymeric nanocapsules containing DEXM were generated using a nanoprecipitation technique in the current work. Oleic acid was chosen as the PNCs’ oil core because it had the highest DEXM solubilizing capacity. The advantages of nanosized drug delivery systems, including uniform particle size distributions with low polydispersity, physical stability, and excellent encapsulation effectiveness, are demonstrated by nanocapsules with oleic acid-core (Torge et al. 2017). Oleic acid’s solubility in the organic phase during the formation of the nanocapsules may cause the high DEXM encapsulation in the oil core (it has a very low hydrophilic-lipophilic balance (HLB) value of 1). In addition, oleic acid has a high partition coefficient (log Kow = 7.64) that avoids partitioning in the aqueous phase (Anarjan et al. 2013; Torge et al. 2017). Similar outcomes were noted by Torge et al. (2017), who manufactured ciprofloxacin nanocapsules using oleic acid as the oil core and reported that employing oleic acid for the creation of nanocapsules led to a greater drug loading and encapsulation efficiency.
During the development procedure, CTAB was introduced to the internal organic phase to create the nanocapsules’ cores. This helped the sodium alginate coat to form around the nanocapsules. Positively charged surfactants may interact ionically with sodium alginate, which is negatively charged, to produce the polymer coat. El-Gogary et al. (2020) created hyaluronan nanocapsules using the CTAB earlier. They examined how negatively charged hyaluronic acid and positively charged CTAB interact ionically to form the cross-linked hyaluronic acid polymer shell.
In a previous study, Lertsutthiwong et al. (2008), turmeric oil was encapsulated into alginate nanocapsules. The coat of nanocapsules was produced by crosslinking of sodium alginate with calcium chloride. The authors concluded that the developed nanocapsules showed good stability and enhanced turmeric oil solubility and activity. Also, calcium chloride was utilized in previous literature to prepare alginate nanocapsule for encapsulation of indomethacin (Fernando et al. 2022). The authors found that the developed alginate nanocapsules that contain sunflower oil showed smaller encapsulation efficiency and large particle size compared with the results obtained in the current study. In the current work, we created DEXM polymeric nanocapsules using sodium alginate, a negatively charged polymer shell, and CTAB, a cationic surfactant. The shell polymer (alginate) concentration was set at 0.05% w/v. When the concentration of sodium alginate was raised, the aqueous phase’s higher viscosity caused an increase in particle size and aggregation.
Further, Liu et al. (2014) prepared drug loaded alginate nanocapsules using calcium ion as a crosslinker, then introduced drug loaded nanoacpsules into alginate fiber as a promising drug delivery for wound dressing.
For nanocapsule polydispersity, P.D.I. values below 0.5 are considered acceptable. They provide evidence of the particles’ homogeneous size distribution and the nanocapsules’ creation (El-Sheridy et al. 2019). As electrostatic repulsion inhibits particle aggregation, the produced alginate nanocapsules showed a negative zeta potential of roughly 30 mV. This discovery indicates that the nanocapsules are stable. The chemical nature of the polymer and surfactant allows us to calculate the zeta potential. The adsorption of alginate on the surface of the nanocapsule may be responsible for the negative charge of DEXM-Alg-NCs. Wang et al. (2010) reported that particles demonstrating zeta potential values equal to or lower than − 30 mV showed a high level of physical stability during storage; thus, zeta-potential is a crucial parameter because it provides the concept of the stability of a nanoparticulate system. Various concentrations of CTAB and hydrophilic surfactants (Tween®80 and Pluronic F-68) were utilized to optimize particle size within the nanoscale range, achieve acceptable P.D.I, maximize EE %, and attain high-value zeta potential. It investigated how changing the ratio of hydrophilic surfactant (Tween®80) to CTAB affected the results (Table 1). As demonstrated, utilizing Pluronic F-68 caused aggregation and precipitation, most likely caused by Tween®superior 80’s stability and stronger adherence properties (Wang et al. 2010). As a result of the charge neutralization effect caused by using higher concentrations of CTAB, the findings additionally revealed that using smaller amounts of CTAB in the development of polymeric nanocapsules is preferable to produce small nanocapsules with high zeta-potential, high EE%, as well as good stability (Table 1). An earlier research found a similar result, reporting that increased CTAB concentrations cause an increase in particle size aggregation (El-Gogary et al. 2020). Also, the increase in Tween 80 concentration from 0.01 to 0.03 g leads to a notable improvement in encapsulation efficiency (Table 1). An increase in hydrophilicity and solubilizing properties most likely explains this discovery. Tween 80 has a high value of HLB of 15 (Anarjan et al. 2013). The SEM examination supported the DLS findings and revealed spherical, non-aggregated nanocapsules, validating the preparation strategy.
Based on the aforementioned experimental results, it was discovered that a mixture of 5 mg CTAB, 0.05% w/v sodium alginate, and 0.03 g Tween 80 generated stable alginate nanocapsules with the lowest P.D.I. (0.22) and smallest size (2877.5 nm) possible (formulation PNC5). PNC5 was chosen as the subject of additional in-vitro and in-vivo research.
FT-IR analysis results verified the development of polymeric nanocapsules, whereas the distinctive DEXM beaks were entirely lost. A significant (p < 0.05) lower cumulative percent (12.34%) of DEXM was released from the chosen alginate nanocapsules after 2 h compared to that (91.51%) from free drug solution at the same time, demonstrating the prolonged release pattern of DEXM with alginate polymeric nanocapsules (Fig. 2 III). The prolonged-release profile may be attributed to sodium alginate’s coating’s ability to operate as a membrane barrier and DEXM’s strong affinity for the oil core of the produced nanocapsules. This result proved that alginate nanocapsules provide prolonged sustained release of DEXM, which is acceptable for long-term treatment and avoids undesirable side effects since it allows the nanoparticulate system to continuously release small amounts of the drug over time.
The stability of nanosized systems is an essential concept of their successful development. The stability of DEXM-Alg-NC5 was monitored for 30 days at 4 o C and 25 o C. The formulation was stable at 4 o C and 25 o C with insignificant (p > 0.05) increase in particle size and PDI (Fig. 3). These findings revealed that the prepared DEXM nanocapsules were stable during storage at room temperature and in the refrigerator. The high stability of the developed alginate nanocapsules may be related to the high negative surface charge, where they retard the aggregation because of repulsive forces (Lobato et al. 2013). The high stability of the nanocapsules probably resulted from the hydrophilic surfactant employed (Tween 80), besides the coating polymer sodium alginate that surrounded the oil core (El-Gogary et al. 2020).
As shown in the results, the blood glucose levels were significantly reduced in the free DEXM-treated and the DEXM polymeric nanocapsules-treated rats compared to the vehicle group. However, this reduction remained within the normal range of blood glucose in rats (85–132 mg/dl), as reported by Kohn et al. (2002). The mean blood glucose level in the DEXM polymeric nanocapsules group (96.3 ± 2.4) was slightly higher than in the free DEXM group (94.6 ± 2). From our point of view, this reduction in blood glucose levels may reflect a reduction in food intake following DEXM-induced gastritis. Lee et al. (2013) and Yan et al. (2021) reported significant reductions in food and water intake in rats with induced chronic gastritis. Noh et al. (2014) documented that DEXM administration to rats led to a significant reduction in food intake. Additionally, Choi et al. (2015) reported a reduction in patients’ blood glucose levels with chronic gastritis, indicating that it directly affects food intake and blood glucose levels.
The considerable drop in body weight observed in the treated rats using free DEXM and DEXM polymeric nanocapsules compared to the vehicle group may be due to the induced gastritis. This can be after the reduction in food intake. Similar findings were made by Liu et al. (2021) and Yan et al. (2021), who found considerable weight loss following the induction of gastritis. Rats given DEXM polymeric nanocapsule treatment showed a reduced weight loss percentage (14% vs. 28%), possibly due to a milder form of induced gastritis.
The relationship between glucocorticoid therapy and stomach ulcer disease, often known as steroid ulcer, is extensively documented. The ulcers’ pathologically soft, flexible, almost rubbery quality has a minimum fibrous reaction. Yet, a thorough analysis of the available clinical data lacks convincing evidence for such a steroid ulcer (Yasir et al. 2022). Therefore, we committed a trial to demonstrate the gastropathic effects of the fixed dose of systemic DEXM polymeric nanocapsules versus free DEXM in rats after a fixed duration of administration. In the stomach tissues of both DEXM-treated groups, some gastropathic indicators, including oxidative insult assessment macroscopic, microscopic, and morphometric examination, were carried out.
Oxidative insults were evident in both DEXM-treated groups, manifested by the significant increment in gastric MDA and reduced GSH levels. DEXM has been implicated in experimental gastric ulcer development by various mechanisms, such as enhancing free radical production and induction of lipid peroxidation. Lipid peroxidation involves the formation of lipid radicals that attack the unsaturated lipids, ultimately destroying cell membranes. Dexamethasone treatment was also associated with reduced enzymatic and non-enzymatic antioxidant defense mechanisms in the gastric tissues (Rizk et al. 2017; Rateb et al. 2021). Dexamethasone triggers basal and drug-induced gastric acid secretion (Swamy et al. 2011) and increases the risk of gastric ulcers by prostaglandin synthase inhibition, thus suppressing the vital gastrointestinal protecting enzyme (Tseng et al. 2015). Substantially, it reduces the stimulation of epidermal growth factors, which enhance the proliferation of stomach epithelial cells (Luo et al. 2007). In addition, DEXM slows epithelial regeneration in experimental gastric ulcers and inhibits angiogenesis in the rat stomach (Luo et al. 2004).
The histopathological results supported the findings of oxidative damage. A gross examination of the stomach samples of the free DEXM-administrated group revealed hemorrhagic ulcers with whitish necrotic foci. These results are similar to those of the study on pregnant animals exposed to DEXM (Leão et al. 2008). Microscopic examination of stomach sections from the free DEXM-administrated group showed necrosis and desquamation of the lining epithelium accompanied by angiopathic lesions characterized by interstitial hemorrhage between gastric glands in lamina propria and edema in the deep layer of gastric mucosa between gastric glands. Furthermore, the morphometric analysis of the mucosal thickness in the free DEXM-administrated group showed a significant reduction in gastric mucosa thickness compared to the vehicle group. This agrees with other study findings that indicated deterioration of the surface epithelium and enlargement of intracellular gaps associated with decreased secretory granules in rats with stomach lesions caused by DEXM (Elitok et al. 2005).
On the contrary, the histopathological examination of the DEXM polymeric nanocapsules-treated group’s stomach showed few numbers of hemorrhagic ulcers characterized by mild desquamation of the gastric epithelium with mild hyperemia, with increased mucosal thickness when compared with the free DEXM-administrated group. This was in line with study findings on how anti-inflammatory drug-delivering solid lipid nanoparticles affected inflammatory bowel disease. It was noted that the administration of DEXM cholesteryl butyrate-solid lipid NPs achieved a significant cytokine decrease compared to the cytokine plasma concentration of the untreated mice with dextran sulfate sodium-induced colitis (Dianzani et al. 2017). In addition, DEXM-loaded lipid nanoemulsions effectively reduced the expression of proinflammatory genes in a mouse model of acute inflammation (Simion et al. 2016).
The mechanism behind the cardiotoxic effect of DEXM is elusive. In a trial to demonstrate the cardiopathic effects of the fixed dose of systemic DEXM polymeric nanocapsules versus free DEXM in rats after a fixed duration of administration, we measured a group of cardiopathic indices as serum CK-MB, LDH, cardiac SOD, and MDA.
Cytosolic creatine kinase (CK) exists in 3 isoforms: MM-CK, expressed in skeletal muscle; MB-CK, expressed in cardiac muscle; and BB-CK, expressed in smooth muscle and most non-muscle tissues such as the brain (Cabaniss 1990). Creatine kinase exhibits some functions in cell energy metabolism. It enables energy storage in phosphocreatine by catalyzing the reversible transfer of high-energy phosphate from ATP to creatine. This additional energy buffer is crucial for maintaining ATP balance in muscle cells (Hettling et al. 2011). Lactate dehydrogenase is an important intracellular enzyme to produce energy. It facilitates the transformation of pyruvate to lactate under anaerobic conditions. Early cardiac injury can be reflected by a rise in LDH, which is used to identify acute myocardial infarction (Zeng et al. 2022). In our study, biomarkers of cardiotoxicity, such as CK-MB and LDH levels, were elevated in both treated groups. In addition, we found that DEXM increased intracellular ROS generation, manifested by the significant imbalance in cardiac MDA and SOD levels. Like LDH, CK–MP, which is abundantly expressed in cardiac muscles, is released into the bloodstream after cardiac injury (Abdel-Wahab et al. 2014).
The histopathological cardiotoxic effects of free DEXM and DEXM polymeric nanocapsules showed that free DEXM treatment caused cytoplasmic vacuolation in most myocardial fibers. At the same time, cardiac lesions were decreased in the DEXM polymeric nanocapsules-treated group. In addition, immunohistochemical examination of the cardiac tissue showed a significant increase of caspase-3 expression in heart muscle, indicating apoptosis compared with the DEXM polymeric nanocapsules group. This finding is consistent with the study investigating the cardiotoxic effect of DEXM-high-dose in rats and the therapeutic effect of carvedilol by clarifying the role of the α1-adrenergic receptor (Mohamed et al. 2022).
In this context, de Salvi Guimarães et al. (2017) noted that DEXM damages cardiac function and structure since its use contributes to pathological cardiac remodeling. Their study demonstrated an increase in the size of hearts treated with DEXM, increased fibrosis, and a reduction in diastolic function and important alterations to the proper functioning of the heart muscle accompanied by alteration in the expression of proteins related to homeostasis of Ca2+. Mohamed et al. (2022) referred to DEXM-induced cardiac injury as increasing oxidative stress and apoptosis accompanied by elevation in cardiac CK-MB and MDA. Dexamethasone’s cardiotoxic side effects were closely tied to its atherogenic side effects and the development of dyslipidemia (Ferraù et al. 2015).
Conclusion
Dexamethasone was successfully encapsulated into polymeric nanocapsules of sodium alginate coating polymer. The developed alginate nanocapsules exhibited desirable parameters regarding particle size, PDI, zeta potential, encapsulation efficiency, sustained in-vitro release, and physical storage stability. Additionally, an in-vivo assessment of the safety profile of DEXM PNCs showed a better profile than the free formulation, considering the gastric and cardiac damage. Polymeric nanocapsules might be considered a potential nanosystem to deliver DEXM systemically in the long term, avoiding the associated side effects of the free formulations.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdel-Wahab BA, Metwally ME, El-Khawanki MM, Hashim AM (2014) Protective effect of captopril against clozapine-induced myocarditis in rats: role of oxidative stress, proinflammatory cytokines and DNA damage. Chemico-Biol Interact 216:43–52
Anarjan N, Tan CP (2013) Effects of selected polysorbate and sucrose ester emulsifiers on the physicochemical properties of astaxanthin nanodispersions. Molecules 18:768–777
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier health sciences
Bandyopadhyay U, Biswas K, Bandyopadhyay D, Ganguly C, Banerjee R (1999) Dexamethasone makes the gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase and peroxidase-two important gastroprotective enzymes. Mol Cell Biochem 202:31–36
Butler E, Møller MH, Cook O, Granholm A, Penketh J et al (2019) The effect of systemic corticosteroids on the incidence of gastrointestinal bleeding in critically ill adults: a systematic review with meta-analysis. Intensive Care Med 45:1540–1549
Cabaniss CD (1990) in Clinical Methods: The History, Physical, and Laboratory Examinations (eds H. K. Walker, W. D. Hall, J. W. Hurst) (Butterworths Copyright © 1990, Butterworth Publishers, a division of Reed Publishing
Chen F, Hao L, Zhu S, Yang X, Shi W et al (2021) Potential adverse effects of Dexamethasone Therapy on COVID-19 patients: review and recommendations. Infect Dis Ther 10:1907–1931
Choi MK, Kang MH, Kim MH (2015) Dietary intake assessment and biochemical characteristics of blood and urine in patients with chronic gastritis. Clin Nutr Res 4:90–96
Covar RA, Leung DY, Mccormick D, Steelman J, Zeitler P et al (2000) Risk factors associated with glucocorticoid-induced adverse effects in children with severe asthma. J Allergy Clin Immunol 106:651–659
De Salvi Guimarães F, De Moraes WM, Bozi LH, Souza PR, Antonio EL et al (2017) Dexamethasone-induced cardiac deterioration is associated with both calcium handling abnormalities and calcineurin signaling pathway activation. Mol Cell Biochem 424:87–98
Deng S, Gigliobianco MR, Censi R, Di Martino P (2020) Polymeric nanocapsules as Nanotechnological Alternative for Drug Delivery System: current status, challenges and opportunities. Nanomaterials (Basel) 10
Dianzani C, Foglietta F, Ferrara B, Rosa AC, Muntoni E et al (2017) Solid lipid nanoparticles delivering anti-inflammatory drugs to treat inflammatory bowel disease: effects in an in vivo model. World J Gastroenterol 23:4200
El Zahaby AA, Abdel Alim A, El Sharawy AF (2017) Role of Rebamipide and \ or pantoprazole in preventing Dexamethasone Induced gastritis in Senile male albino rats %J. Egypt J Hosp Med 67:789–805
El-Gogary RI, Khattab MA, Abd-Allah H (2020) Intra-articular multifunctional celecoxib loaded hyaluronan nanocapsules for the suppression of inflammation in an osteoarthritic rat model. Int J Pharm 583:119378
El-Lekawy AM, Abdallah DM, El-Abhar HS (2019) Alanyl-glutamine Heals Indomethacin-induced gastric ulceration in rats Via Antisecretory and anti-apoptotic mechanisms. J Pediatr Gastroenterol Nutr 69:710–718
El-Sheridy NA, Ramadan AA, Eid AA, El-Khordagui LK (2019) Itraconazole lipid nanocapsules gel for dermatological applications: in vitro characteristics and treatment of induced cutaneous candidiasis. Colloids Surf B Biointerfaces 181:623–631
Elitok B, Elitok Ö, Ketani M, Kurt D, Kanay Z (2005) The effect of dexamethasone on gastric mucosal changes following sialoadenectomy in rat. J Endocrinol Investig 28:700–703
Fernando IPS, Kirindage KGIS, Jayasinghe AMK, Han EJ, Lee C-M et al (2022) Alginate nanocapsules by water-in-oil emulsification and external gelation for drug delivery to fine dust stimulated keratinocytes. Int J Biol Macromol 218:102–114
Ferraù F, Korbonits M (2015) Metabolic comorbidities in Cushing’s syndrome. Eur J Endocrinol 173:M133–157
Fichter M, Baier G, Dedters M, Pretsch L, Pietrzak-Nguyen A et al (2013) Nanocapsules generated out of a polymeric dexamethasone shell suppress the inflammatory response of liver macrophages. Nanomedicine 9:1223–1234
Fraguas-Sánchez AI, Torres-Suárez AI, Cohen M, Delie F, Bastida-Ruiz D et al (2020) PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and In Ovo Assessment. Pharmaceutics 12
Guslandi M (2013) Steroid ulcers: any news? World J Gastrointest Pharmacol Ther 4:39–40
Hatahet T, Morille M, Shamseddin A, Aubert-Pouëssel A, Devoisselle J et al (2017) Dermal quercetin lipid nanocapsules: influence of the formulation on antioxidant activity and cellular protection against hydrogen peroxide. Int J Pharm 518:167–176
Hettling H, Van Beek JH (2011) Analyzing the functional properties of the creatine kinase system with multiscale ‘sloppy’ modeling. PLoS Comput Biol 7:e1002130
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL et al (2021) Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704
Hsiang KW, Ng YY, Lu CL, Chen TS, Lin HY et al (2010) Corticosteroids therapy and peptic ulcer disease in nephrotic syndrome patients. Br J Clin Pharmacol 70:756–761
Jia M, Deng C, Luo J, Zhang P, Sun X et al (2018) A novel dexamethasone-loaded liposome alleviates rheumatoid arthritis in rats. Int J Pharm 540:57–64
Jirak P, Van Almsick V, Dimitroulis D, Mirna M, Seelmaier C et al (2022) Dexamethasone improves Cardiovascular outcomes in critically ill COVID-19, a Real World Scenario Multicenter Analysis. Front Med (Lausanne) 9:808221
Kiss EL, Berkó S, Gácsi A, Kovács A, Katona G et al (2020) Development and characterization of potential ocular mucoadhesive Nano lipid carriers using full Factorial Design. Pharmaceutics 12.
Klippstein R, Wang JT, El-Gogary RI, Bai J, Mustafa F et al (2015) Passively targeted curcumin-loaded PEGylated PLGA nanocapsules for Colon cancer therapy in vivo. Small 11:4704–4722
Kohn DF, Clifford CB (2002) Biology and Diseases of Rats. (Laboratory Animal Medicine.:121– 65. https://doi.org/10.1016/B978-012263951-7/50007-7. Epub 2007 Sep 2.)
Kumari S, Dandamudi M, Rani S, Behaeghel E, Behl G et al (2021) Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease. Pharmaceutics 13
Leão P, Oliveira M, Sousa N (2008) The morphologic changes in the Gastric Wall Associated with prenatal DEXM Administration
Lee SE, Song HJ, Park SY, Nam Y, Min CH et al (2013) Effect of ECQ on Iodoacetamide-Induced Chronic gastritis in rats. Korean J Physiol Pharmacol 17:469–477
Lertsutthiwong P, Noomun K, Jongaroonngamsang N, Rojsitthisak P, Nimmannit U (2008) Preparation of alginate nanocapsules containing turmeric oil. Carbohydr Polym 74:209–214
Liu L, Jiang L, Xu G, Ma C, Yang X et al (2014) Potential of alginate fibers incorporated with drug-loaded nanocapsules as drug delivery systems. J Mater Chem B 2:7596–7604
Liu S, Su Z, Zhang J, Fan Q, Gao J et al (2021) Dynamic observation of the progression of chronic gastritis to gastric cancer in a disease–TCM pattern rat model. J Traditional Chin Med Sci 8:124–134
Lobato KB, Paese K, Forgearini JC, Guterres SS, Jablonski A et al (2013) Characterisation and stability evaluation of bixin nanocapsules. Food Chem 141:3906–3912
Luo JC, Shin VY, Liu ES, Ye YN, Wu WK et al (2004) Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur J Pharmacol 485:275–281
Luo J-C, Chi C-W, Lin H-Y, Chang F-Y, Lu C-L et al (2007) Dexamethasone inhibits epidermal growth factor-stimulated gastric epithelial cell proliferation. J Pharmacol Exp Ther 320:687–694
Macallister CG, Andrews FM, Deegan E, Ruoff W, Olovson SG (1997) A scoring system for gastric ulcers in the horse. Equine Vet J 29:430–433
Marchiori M, Lubini G, Dalla Nora G, Friedrich R, Fontana M et al (2010) Hydrogel containing dexamethasone-loaded nanocapsules for cutaneous administration: preparation, characterization, and in vitro drug release study. Drug Dev Ind Pharm 36:962–971
Mo Z, Ban J, Zhang Y, Du Y, Wen Y et al (2018) Nanostructured lipid carriers-based thermosensitive eye drops for enhanced, sustained delivery of dexamethasone. Nanomed (Lond) 13:1239–1253
Mohamed R, Ahmad EA, Omran BHF, Sakr AT, Ibrahim I et al (2022) Carvedilol ameliorates dexamethasone-induced myocardial injury in rats independent of its action on the α1-adrenergic receptor. Naunyn Schmiedebergs Arch Pharmacol 395:1537–1548
Narum S, Westergren T, Klemp M (2014) Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open 4:e004587
Noh KK, Chung KW, Choi YJ, Park MH, Jang EJ et al (2014) β-Hydroxy β-methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS ONE 9:e102947
Pan X, Liu X, Zhuang X, Liu Y, Li S (2020) Co-delivery of dexamethasone and melatonin by drugs laden PLGA nanoparticles for the treatment of glaucoma. J Drug Deliv Sci Technol 60:102086
Rateb E, Khowailed A, Rashed L, Naeem A, Mohammed M (2021) Effect of vitamin D on Dexamethasone Induced metabolic disturbance and gastric Ulcer. Annals Romanian Soc Cell Biology 25:15200–15221
Rizk FH, Ibrahim MA, Abd-Elsalam MM, Soliman NA et al (2017) Gastroprotective effects of montelukast and Nigella sativa oil against corticosteroid-induced gastric damage: they are much more than antiasthmatic drugs. Can J Physiol Pharmacol 95:714–720
Schimmer BP, Funder JW (2011) ACTH, adrenal steroids, and pharmacology of the adrenal cortex. Goodman and Gilman’s the pharmacological basis of therapeutics, 12th edn. McGraw-Hill, New-York, pp 1209–1235
Simion V, Constantinescu CA, Stan D, Deleanu M, Tucureanu MM et al (2016) P-selectin targeted dexamethasone-loaded lipid nanoemulsions: a novel therapy to reduce vascular inflammation. Mediators of inflammation 2016.
Suvarna KS, Layton C, Bancroft JD (2018) Elsevier health sciences,. Bancroft’s theory and practice of histological techniques E-Book
Swamy AH, Sajjan M, Thippeswamy AH, Koti BC, Sadiq AJ (2011) Influence of proton pump inhibitors on dexamethasone-induced gastric mucosal damage in rats. Indian J Pharm Sci 73:193–198
Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P et al (2005) Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J Biol Chem 280:9409–9415
Torge A, Wagner S, Chaves PS, Oliveira EG, Guterres SS et al (2017) Ciprofloxacin-loaded lipid-core nanocapsules as mucus penetrating drug delivery system intended for the treatment of bacterial infections in cystic fibrosis. Int J Pharm 527:92–102
Tseng CL, Chen YT, Huang CJ, Luo JC, Peng YL et al (2015) Short-term use of glucocorticoids and risk of peptic ulcer bleeding: a nationwide population-based case-crossover study. Aliment Pharmacol Ther 42:599–606
Ummanni R, Lehnigk U, Zimmermann U, Woenckhaus C, Walther R et al (2010) Immunohistochemical expression of caspase-1 and– 9, uncleaved caspase-3 and– 6, cleaved caspase-3 and– 6 as well as Bcl-2 in benign epithelium and cancer of the prostate. Exp Ther Med 1:47–52
Wang MT, Jin Y, Yang YX, Zhao CY, Yang HY et al (2010) In vivo biodistribution, anti-inflammatory, and hepatoprotective effects of liver targeting dexamethasone acetate loaded nanostructured lipid carrier system. Int J Nanomed 5:487–497
Yamaguchi M, Niimi A, Minakuchi M, Matsumoto H, Shimizu K et al (2007) Corticosteroid-induced myopathy mimicking therapy-resistant asthma. Annals Allergy Asthma Immunol 99:371–374
Yan Z, Xu T, Xu Y, Chen W, An Z et al (2021) Jianpiyiqi formula ameliorates chronic atrophic gastritis in rats by modulating the Wnt/β-catenin signaling pathway. Exp Ther Med 22:878
Yasir M, Goyal A, Bansal P, Sonthalia S (2022) (StatPearls Publishing, Treasure Island (FL)
Yu A, Shi H, Liu H, Bao Z, Dai M et al (2020) Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int J Pharm 575:118943
Zeng Y, Zhao Y, Dai S, Liu Y, Zhang R et al (2022) Impact of lactate dehydrogenase on prognosis of patients undergoing cardiac surgery. BMC Cardiovasc Disord 22:404
Acknowledgements
The study’s authors express their sincere gratitude for all the support they received in completing their work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Rania (A) Abdel-Emam: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Review, editing. Rasha (B) Abd-Ellatief: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing - original draft. Marwa F. Ali: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing - original draft, Review, editing. Abeer S. Hassan: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing - original draft, Review, editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors (R.A. Abdel-Emam, M.F. Ali, A.S. Hassan, and R.B. Abd-Ellatief) declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Statement of animal rights
“All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.” “All procedures performed in the study were in accordance with the ethical standards of Institutional Animal Care and Use Committee (IACUC) in Assiut University, Faculty of Medicine, (IACUC approval No. (IRB: 04-2023-300100)”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Emam, R.A., Ali, M.F., Hassan, A.S. et al. Development and evaluation of dexamethasone-loaded bioadhesive polymeric nanocapsules for mitigating cardiac and gastric adverse effects of free dexamethasone. J. Pharm. Investig. (2024). https://doi.org/10.1007/s40005-024-00686-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40005-024-00686-7