Abstract
Purpose
Febuxostat is non-purine, selective inhibitor of xanthine oxidase for treatment of gout. It exhibits poor bioavailability. The goal of the present study was to enhance the oral bioavailability of Febuxostat through β-Cyclodextrin nanosponges, subsequently reduce the dose and side effects.
Methods
Nanosponges were formed by cross-linking β-Cyclodextrin with carbonate bonds using different molar ratio (1:4, 1:6, 1:8 and 1:10 β-Cyclodextrin: crosslinker). Drug was incorporated by solvent evaporation method. Nanosponge formulations were evaluated and formulations that released (≥ 30%) at first hour followed by controlling the release (≥ 75%) at 6 h were further evaluated and tableted by direct compression. The optimum tablet formulations based upon drug release were investigated for accelerated stability testing and for comparative bioavailability with a marketed product.
Results
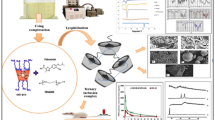
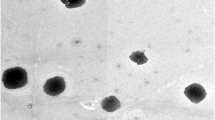
SEM illustrates porous and sponge like structure. DSC and FTIR studies confirmed the formation of nanosponges and encapsulation of Febuxostat within it. The zeta-potentials were high (− 21.5 to − 32.3 mV). The particle sizes were between 224.7 and 305.6 nm. The in vitro release study showed a biphasic release pattern. Oral bioavailability of selected formulation and marketed product showed enhanced Cmax (1655.0 ± 18.5 vs. 1592.7 ± 95.9 ng/mL) and AUC0−∞ (14,576.7 ± 1681.7 vs. 6449.7 ± 677.1 ng h/mL). The relative bioavailability was found to be 217.9%.
Conclusion
Nanosponges is a feasible approach to improve the oral bioavailability of Febuxostat.






Similar content being viewed by others
References
Ahuja BK, Jena SK, Paidi A, Bagri S, Suresh S (2015) Formulation, optimization and in vitro–in vivo evaluation of Febuxostat nanosuspension. Int J Pharm 478:540–552
Anandam S, Selvamuthukumar S (2014) Fabrication of Cyclodextrin nanosponges for Quercetin delivery: physicochemical characterization, photostability, and antioxidant effects. J Mater Sci 49:8140–8853
Asif U, Sherwani AK, Akhtar N, Shoaib MH, Hanif M et al (2015) Formulation development and optimization of Febuxostat tablets by direct compression method. Adv Polym Tech 35:129–135
British pharmacopoeia (2013) The stationary office, 7th edn. Volume V, Appendix XII and XVII. Cartwright AC, London.
Caldera F, Tannous M, Cavalli R, Zanetti M, Trotta F (2017) Evolution of Cyclodextrin nanosponges. Int J Pharm 531:470–479
Darwish MKM, Abu El-Enin ASM, Mohammed KHA (2019) Formulation, optimization, and evaluation of raft forming formulations containing Nizatidine. Drug Dev Ind Pharm 45:651–663
Dash S, Murthy PN, Nath L, Chowdhury P (2010) Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 67:217–223
Dubey P, Sharma HK, Shah S, Tyagi CK, Chandekar AR et al (2016) Formulations and evaluation of Cyclodextrin complexed Ceadroxil loaded nanosponges. Int J Drug Deliv 9:84–100
Gide M, Sharma P, Saudagar R, Shrivastava B (2014) Method development and validation for determination of Febuxostat from spiked human plasma using RP-HPLC with UV detection. Chromatogr Res Int. https://doi.org/10.1155/2014/307430
Han X, Qi W, Dong W, Guo M, Ma P et al (2016) Preparation, optimization and in vitro–in vivo investigation for capsules of the choline salt of Febuxostat. Asian J Pharm 11:715–721
Kiliçarslan M, Baykara T (2003) The effect of the drug/polymer ratio on the properties of the Verapamil HCl loaded microspheres. Int J Pharm 252:99–109
Kumar S, Pooja TF, Rao R (2018) Encapsulation of Babchi oil in Cyclodextrin-based nanosponges: physicochemical characterization, photodegradation, and in vitro cytotoxicity studies. Pharmaceutics 10:1–18
Mady FM, Ibrahim SRM (2018) Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of Ellagic acid. Pak J Pharm Sci 31:2069–2076
Rao MRP, Bhingone RC (2015) Nanosponge-based pediatric-controlled release dry suspension of Gabapentin for reconstitution. Drug Dev Ind Pharm 41:1–8
Rao M, Bajaj A, Khole I, Munjapara G, Trotta F (2013) In vitro and in vivo evaluation of β- Cyclodextrin based nanosponges of Telmisartan. J Incl Phenom Macrocycl Chem 77:135–145
Shende PK, Trotta F, Gaud RS, Deshmukh K, Cavalli R et al (2012) Influence of different techniques on formulation and comparative characterization of inclusion complexes of ASA with β-Cyclodextrin and inclusion complexes of ASA with PMDA cross-linked β-Cyclodextrin nanosponges. J Incl Phenom Macrocycl Chem 74:447–454
Shringirishi M, Mahor A, Gupta R, Prajapati SK, Bansal K et al (2017) Fabrication and characterization of Nifedipine loaded β-Cyclodextrin nanosponges: an in vitro and in vivo evaluation. J Drug Deliv Sci Technol 41:344–350
Singha P, Rena X, Guoa T, Wua L, Shakyaa S et al (2018) Biofunctionalization of β-Cyclodextrin nanosponges using Cholesterol. Carbohydr Polym 190:23–30
Szejtli J (1998) Introduction and general overview of Cyclodextrin chemistry. Chem Rev 98:1743–1754
United States Pharmacopeia (USP XXV) (2010–2012) NF 20, united states, Pharmacopieal convention, Inc., 12601, Twin brook parkway, Rockville, M.D 20852, Asian Ed., pp 62–63
Venuti V, Rossi B, Mele A, Melone L, Punta C et al (2017) Tuning structural parameters for the optimization of drug delivery performance of Cyclodextrin-based nanosponges. Expert Opin Drug Deliv 14:331–340
Zhang JX, Ma PX (2013) Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv Drug Deliv Rev 65:1215–1233
Acknowledgements
I would like to thank Dr Maha khalifa Ahmed (Lecturer of pharmaceutics department) for supporting me. I would like to give a privilege to all members of the faculty of pharmacy, Al-Azhar University for providing excellent facilities and deep support for carrying out the research work, specially (Organic Chemistry Department for help in the synthesis of nanosponges). I am thankful to EIPICO (Egypt) for generous donation of β-CD and grateful to Egyptian Petroleum Research Institute for the particle size and zeta potential work and Central Laboratory (Cairo University) for help in the DSC and FTIR work. I am thankful to Al-Roaa Laboratory for the in vivo work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Statement of human and animal rights
All institutional and national guidelines for the care and use of laboratory animals were followed. Measurement of pharmacokinetic parameters of the treatments conformed to guidelines of Institutional Animal Ethical of Faculty of Pharmacy, Al-Azhar University (Approval number: 196).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amin, O.M., Ammar, A. & Eladawy, S.A. Febuxostat loaded β-cyclodextrin based nanosponge tablet: an in vitro and in vivo evaluation. J. Pharm. Investig. 50, 399–411 (2020). https://doi.org/10.1007/s40005-019-00464-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-019-00464-w