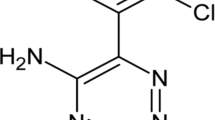
Abstract
The objective of the present investigation was to prepare phenytoin microemulsion for intranasal administration by application of experimental design. Phenytoin microemulsion was prepared by water titration method. The concentration of oil (X1), surfactant (X2) and cosurfactant (X3) were selected as independent variables, in a simplex centroid design, from microemulsion region obtained from pseuodoternary phase diagram while the globule size (Y1) and cumulative phenytoin diffused at 60 min (Y2) through sheep nasal mucosa were taken as dependent variables. Mathematical models were generated for the response variables. The validity of the generated equations was established using check point batches. Microemulsion that had a smaller globule size of dispersed phase and high level of % drug diffusion was desirable. The generated mathematical equations were used to identify the desirable zone where all the criteria for the responses were satisfied and an optimum batch was recognized. Ex-vivo study of optimum microemulsion on sheep nasal mucosa shows absence of nasal toxicity. It was found that faster recovery from seizures was obtained in rats treated with intra nasal phenytoin microemulsion in comparison to the rats treated with oral microemulsion and nasal solution. Higher concentration of phenytoin was found in rats treated with intranasal microemulsion in comparison to the rats treated with phenytoin solution administered intraperitoneally. Gamma scintigraphy results also suggested faster availability of drug into brain when microemulsion was administered intranasally.









Similar content being viewed by others
References
http://www.jmp.com/support/help/Mixture_Designs.shtml#84106. Assessed on 26 Nov 2014
Bandivadeka MM, Pancholi SS, Kaul-Ghanekar R, Choudhari A, Koppikar S (2012) Self-microemulsifying smaller molecular volume oil (Capmul MCM) using non-ionic surfactants: a delivery system for poorly water-soluble drug. Drug Dev Ind Pharm 38(7):883–892
Bragagni M, Mennini N, Furlanetto S, Orlandini S, Ghelardini C, Mura P (2014) Development and characterization of functionalized niosomes for brain targeting of dynorphin-B. Eur J Pharm Biopharm 87(1):73–79
Browne TR, LeDuc B (1995) Phenytoin: chemistry and biotransformation. In: Levy RH, Mattson RH, Meldrum BS (eds) Antiepileptic Drugs, vol 4. Raven Press, New York, pp 283–300
Budavari S (ed) (1995) The Merck index, l2 edn. Merck & Co, Rahway, p 175
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219
Gao ZG, Choi HG, Shin HJ (1998) Physicochemical characterization and evaluation of a ME system for oral delivery of cyclosporin A. Int J Pharm 161:75–86
Jain K, Kumar RS, Sood S, Dhyanandhan G (2013) Betaxolol hydrochloride loaded chitosan nanoparticles for ocular delivery and their anti-glaucoma efficacy. Curr Drug Deliv 10:493–499
Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K (2008) Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm 358:285–291
Lianli N, Iand Kim KH (2002) Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. Int J Pharm 237:77–85
Lindenberg M, Kopp S, Dressman JB (2004) Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm 58(2):265–278
McNamara JO (2001) Chapter 21. Drugs effective in the therapy of the epilepsies. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th ed. Hardman JG, Limbird LE, Gilman AG, eds. McGraw-Hill, New York, pp. 521-547
Patil S, Babbar A, Mathr R, Mishra A, Sawant K (2010) Mucoadhesive chitosan microspheres of carvedilol for nasal administration. J Drug Target 18:321–331
Piao HM, Balkrishnan P, Cho HJ, Kim H, Kim YS, Chung SJ, Shim CK, Kim DD (2010) Preparation and evaluation of fexofenadine microemulsions for intranasal delivery. Int J Pharm 395:309–316
Porecha S, Shah T, Jogani V, Naik S, Misra A (2009) Microemulsion based intranasal delivery system for treatment of insomnia. Drug Deliv 16:128–134
Sweetman SC (2005) Martindale—the complete drug reference, 34th edn. Pharmaceutical Press, London, pp 370–376
Tozer TN, Winter ME (1992) Phenytoin. In: William EE, Jerome JS, William JJ (eds) Applied pharmacokinetics: principles of therapeutic drug monitoring. Applied Therapeutics Inc., Washington, pp 1–44
Van Vliet EA, Schaik R, Edelbroek PM, Redeker AMJ, Aronica E, Wadman WJ, Marchi N, Vezzani A, Gorter JA (2006) Inhibition of multidrug transporter P-glycoprotein improves seizure control in phenytoin-treated chronic epileptic rats. Lubmb Life 47:672–680
Wigent RJ (2005) Chemical kinetics. In: Gennaro AR (ed) Remingtons’ pharmaceutical sciences, 21st edn. Mack Publishing Co., Pennsylvania, pp 266–279
Woodbury JW, Woodbury DM (1991) Vagal stimulation reduces the severity of maximal electroshock seizures in intact rats: use of a cuff electrode for stimulating and recording. Pacing Clin Electrophysiol 14:94–107
Acknowledgments
All authors (S. P. Acharya, K. Pundarikakshudu, P. Upadhyay, P. Shelat, A. Lalwani) declare that they have no conflict of interest. The authors are thankful to Gujarat Council on Science and Technology for providing financial assistance for the project GUJCOST/MRP/201594/10-11/3765.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acharya, S.P., Pundarikakshudu, K., Upadhyay, P. et al. Development of phenytoin intranasal microemulsion for treatment of epilepsy. Journal of Pharmaceutical Investigation 45, 375–384 (2015). https://doi.org/10.1007/s40005-015-0190-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-015-0190-3