Abstract
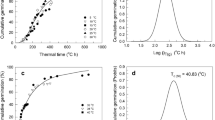
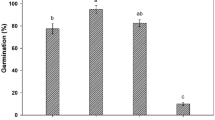
This study examines the role of various environmental factors, viz. salinity, water potential, alcohol, potassium nitrate, gibberellic acid, and root exudates of neighbouring Lolium angustifolius species on seed germination, and root growth of annual ryegrass (L. rigidum). According to a statistical model, a total of 20% seed was germinated at 100 mM of sodium chloride solutions suggesting that the species is moderate to high levels of salinity tolerant. Moderate to high levels of water stress did not inhibit seed germination of L. rigidum, and 15% seeds still germinated at − 8 MPa water. Germination process of non-germinated seeds due to salt stress was not accelerated by gibberellic acid, but about 30% seed germination was increased by 0.02 M potassium nitrate treatment. The hormesis model suggests that concentrations of potassium nitrate more than 0.04 had no effect to enhance seed germination. Seeds of L. rigidum were successfully germinated under a temperature range between 10 and 30 °C. A separate laboratory experiment determined that germination was not affected by root exudates of L. angustifolius but had a significant effect on seedling roots of L. rigidum, where density by variety had a major effect on the root growth of L. rigidum. At 20 seedlings of L. angustifolius, about 80% root growth of L. rigidum was controlled and 60% roots of L. rigidum showed bent or curved growth. These results showed that L. rigidum may possess a wide range adaptive mechanism to different environmental stress.






Similar content being viewed by others
References
Abdel-Farid IB, Kim HK, Choi YH, Verpoorte R (2007) Metabolomic characterization of Brassica rapa leaves by NMR spectroscopy. J Agric Food Chem 55:7936–7943
Arshad MWT, Jr Frankenberger (2002) Ethylene: agricultural sources and applications. Kluwer Academic, New York
Asaduzzaman M, An M, Pratley JE, Luckett DJ, Lemerle D (2014) Canola (Brassica napus) germplasm shows variable allelopathic effects against annual ryegrass (Lolium rigidum). Plant Soil 380(1&2):47–56
Asaduzzaman M, An M, Pratley JE, Luckett DJ, Lemerle D, Coombes N (2016) The seedling root response of annual ryegrass (Lolium rigidum) to neighbouring seedlings of a highly-allelopathic canola (Brassica napus). Flora 219:18–24
Bais HP, Weir TL, Perry LG, Gilory S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev 57:233–266
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego
Belz RG, Hurle K (2005) Differential exudation of two benzoxazinoids one of the determining factors for seedling allelopathy of Triticeae species. J Agric Food Chem 53:250–261
Bennett RN, Rosa EAS, Mellon FA, Kroon PA (2006) Ontogenic profiling of glucosinolates, flavonoids and other secondary metabolites in Eruca sative, Diplotaxis erucoides, Diplotaxis tenuifolia and Bunias orientalis. J Agric Food Chem 54:4005–4015
Brain P, Cousens R (1989) An equation to describe dose responses where there is stimulation of growth at low doses. Weed Res 29:93–96
Chauhan BS, Johnson DE (2010) The role of seed ecology in improving weed management strategies in the tropics. Adv Agron 105:221–262
Chauhan BS, Gill G, Preston C (2006) Tillage system effects on weed ecology, herbicide activity and persistence: a review. Aust J Exp Agric 46:1557–1570
Ding QL, Dang XM, Zhan YF (2007) Effect of potassium nitrate and gibberellin solution soaking on mini-watermelon seed germination. J South China Univ Trop Agric 13:14–16
Gao N, Cui GF, Lai YQ, Zhang SX, Li J, Wang JH, Liu FH (2011) Effects of different treatments on the germination of oriental lily seeds. Acta Agric Univ Jiangxiensis 33:660–664
Goodall J, Witkowski EF, Ammann S, Reinhardt C (2010) Does allelopathy explain the invasiveness of Campuloclinium macrocephalum (pompom weed) in the South African grassland biome? Biol Innov 12:3497–3512
Gupta SM, Pandey P, Grover A, Ahmed Z (2011) Breaking seed dormancy in Hippophaes alicifolia, a high value medicinal plant. Physiol Mol Biol Plants 17:403–406
Hendricks SB, Taylorson RB (1974) Promotion of seed germination by nitrate, nitrite, hydroxylamine, and ammonium salts. Plant Physiol 54:304–309
Huang B, Johnson JW (1995) Root respiration and carbohydrate status of two wheat genotypes in response to hypoxia. Ann Bot 75:427–432
Huang B, Johnson JW, NeSmith S, Bridge DC (1994) Root and shoot growth of wheat genotypes in response to hypoxia and subsequent resumption of aeration. Crop Sci 34:1538–1544
Huang B, Johnson JW, NeSmith S, Bridge DC (1994) Growth physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. J Exp Bot 45:193–202
Lemerle D, Tang HY, Murray GM, Morris S (1996) Survey of weeds and diseases in cereal crops in the southern wheat belt of New South Wales. Aust J Exp Agric 36:545–554
Lemerle D, Blackshaw R, Potter T, Marcroft S, Barrett-Lennard R (1999) Incidence of weeds in canola crops across southern Australia. In: 10th international rapeseed congress, Canberra, Australia
Mahall BE, Callaway RM (1991) Root communication among desert shrubs. Proc Natl Acad Sci USA 88:874–876
Mahall BE, Callaway RM (1996) Effects of regional origin and genotype on intraspecific root communication in the desert shrub Ambrosia dumosa (Asteraceae). Am J Bot 83:93–98
Pujol JA, Calvo JF, Díaz LR (2000) Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Ann Bot 85:279–286
Qu XX, Huang ZY, Baskin JM, Baskin CC (2008) Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte. Ann Bot 2(1):293–299
Radosevich S, Holt J, Ghersa C (1996) Physiological aspects of competition. In: Radosevich S, Holt J, Ghersa C (eds) Weed ecology: implications for management, 2nd edn. Wiley, New York, pp 217–287
Ramzan A, Hafiz IA, Ahmad T, Abbasi NA (2010) Effect of priming with potassium nitrate and dehusking on seed germination of gladiolus (Gladiolus alatus). Pakistan J Bot 42:247–258
Rice EL (1984) Allelopathy, 2nd edn. Academic, Orlando
Ritz C, Streibig JC (2005) Bioassay analysis using R. J Stat Softw 12:1–22
Sarkar D (2008) Lattice: multivariate data visualization with R. Springer, New York
Seefeldt SS, Jensen JE, Fuerst EP (1995) Log-logistic analysis of herbicide dose–response relationships. Weed Technol 9:218–227
Semchenko M, John EA, Hutchings MJ (2007) Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol 176:644–654
Toole EH, Hendricks SB, Borthwick HA, Toolf K (1956) Physiology of seed germination. Ann Rev Plant Physiol 7:1–9
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Williams MM, Schutte BJ, So YF (2012) Maternal corn environment influences wild-proso millet (Panicum miliaceum) seed characteristics. Weed Sci 60:69–74
Wu H, Pratley JE, Lemerle D, Haig T, Verbeek B (1998) Differential allelopathic potential among wheat accessions to annual ryegrass. In: Proceedings of the 9th Australian agronomy conference. The Australian Society of Agronomy
Wu H, Pratley JE, Lemerle D, Haig T (2000) Laboratory screening for allelopathic potential of wheat (Triticum aestivum) accessions against annual ryegrass (Lolium rigidum). Aust J Agric Res 51:259–266
Yu Q, Cairns A, Powles SB (2004) Paraquat resistance in a population of Lolium rigidum. Funct Plant Biol 31:247–254
Yücel E, Yilmaz G (2009) Effects of different alkaline metal salts (NaCl, KNO3), acid concentrations (H2SO4) and growth regulator (GA3) on the germination of Salvia cyanescens Boiss. and Bal. seeds. Gazi Univ J Sci 22:123–127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, A., Asaduzzaman, M. Statistical Modelling of Seed Germination and Seedlings Root Response of Annual Ryegrass (Lolium rigidum) to Different Stress. Agric Res 8, 262–269 (2019). https://doi.org/10.1007/s40003-018-0379-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-018-0379-6