Abstract
Purpose
The objective examination of the Post-COVID syndrome (PCS) remains difficult due to heterogeneous definitions and clinical phenotypes. The aim of the study was to verify the functionality and correlates of a recently developed PCS score.
Methods
The PCS score was applied to the prospective, multi-center cross-sectoral cohort (in- and outpatients with SARS-CoV-2 infection) of the "National Pandemic Cohort Network (NAPKON, Germany)". Symptom assessment and patient-reported outcome measure questionnaires were analyzed at 3 and 12 months (3/12MFU) after diagnosis. Scores indicative of PCS severity were compared and correlated to demographic and clinical characteristics as well as quality of life (QoL, EQ-5D-5L).
Results
Six hundred three patients (mean 54.0 years, 60.6% male, 82.0% hospitalized) were included. Among those, 35.7% (215) had no and 64.3% (388) had mild, moderate, or severe PCS. PCS severity groups differed considering sex and pre-existing respiratory diseases. 3MFU PCS worsened with clinical severity of acute infection (p = .011), and number of comorbidities (p = .004). PCS severity was associated with poor QoL at the 3MFU and 12MFU (p < .001).
Conclusion
The PCS score correlated with patients’ QoL and demonstrated to be instructive for clinical characterization and stratification across health care settings. Further studies should critically address the high prevalence, clinical relevance, and the role of comorbidities.
Trail registration number
The cohort is registered at www.clinicaltrials.gov under NCT04768998.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Three years after the 2019 coronavirus (COVID-19) pandemic, the long-term consequences of COVID-19 in terms of prognosis, quality of life and ability to work have become the focus of medical research [1, 2]. The World Health Organization (WHO) has defined the Post-COVID syndrome (PCS), also known as Long-COVID or Post-COVID condition (PCC), as a condition that may affects patients after severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection and results in symptoms that appear or persist three months after diagnosis, last for at least two months, and cannot be explained by any other diagnosis [3]. Other definitions also exist, e.g., from the UK National Institute for Health and Care Excellence (NICE) [4] and the Centers for Disease Control and Prevention (CDC) [5]. As most PCS studies do not adhere to one of these definitions, they contribute to further heterogeneity by using a diverse set of symptoms and time points [6, 7]. Given diverse diagnostic criteria and clinical phenotypes, there is a significant lack of objective and standardized measures to investigate the pathogenesis, prevalence, and effective treatment of PCS.
To complicate matters, there are difficulties in applying these broad criteria to existing cohort studies designed in the early phase of the pandemic, when PCS was neither widely known nor well defined among researchers. Currently, researchers are searching for existing data suggesting PCS based on different study designs (follow-up time points, duration of symptoms) and using symptom clusters of varying magnitude in their respective cohorts [6,7,8,9]. In addition, it is difficult, but important, to disentangle the causal impact of the COVID-19 infection from other pandemic-related illnesses (e.g. psychiatric disorders due to social isolation) [10, 11] or pre-existing comorbidities [12]. This need is emphasized by the WHO, which limits itself to symptoms for which there is "no other explanation" [3]. The diversity of symptoms in PCS raises the question of whether there are distinct clinical phenotypes [13]. Researchers propose different clinical profiles, symptom clusters, or scoring systems [13,14,15,16], leading to challenges in comparability and assessing PCS quantification and clinical relevance.
At the beginning of the COVID-19 pandemic, Germany established the “Network of University Medicine (NUM)” to coordinate and support national research on COVID-19. As part of this effort, the “National Pandemic Cohort Network (NAPKON)” was created. NAPKON is a multi-center prospective observational cohort study consisting of three cohorts with comprehensive long-term follow-up schemes [17]. With the shift in research priorities towards PCS, the NAPKON cohorts require a standardized, easily applicable PCS definition to further investigate the pathogenesis of PCS. Bahmer et al. (2022) developed the Post-COVID syndrome score (PCS score) based on the data of the population-based cohort in NAPKON (“Populationsbasierte Platform”, POP), providing a useful tool for evaluating the presence and severity of PCS symptoms [14].
Objective
Our aim was to implement the PCS score developed by Bahmer et al. in the cross-sectoral cohort (“Sektorenübergreifende Plattform”, SUEP) of NAPKON and to help standardize and objectify the definition of symptom-based PCS across the network. To achieve this, we calculated the symptom complexes-based PCS score using two approaches: firstly, we mapped the symptom complexes only to patient information obtained during follow-up visits at 3 and 12 months after initial infection. Secondly, we utilized Patient Reported Outcome Measures (PROMs) to identify the occurrence of individual symptoms. We analyzed the frequency of the PCS score severity groups stratified by relevant demographic, disease-related information and risk factors and compared PCS score severity with the QoL outcomes. Additionally, we sought to obtain an initial assessment of the relationship between the PCS score and functional and social impairments and evaluated changes in the PCS score over time. These results are critically discussed in the context of other PCS definitions/scores and relevant challenges in the field.
Methods
Study design and sample
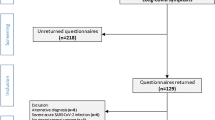
The SUEP cohort of NAPKON recruited SARS-CoV-2 positive tested patients and controls of all ages in all sectors of health care (university hospitals, non-university hospitals, and primary care practices) in Germany, encompassing in- and outpatients since November 4, 2020. The SUEP collects primary health record data, basic clinical phenotyping information, imaging data, bio samples, and PROMs at 57 study sites. The key inclusion criterion was a positive polymerase chain reaction (PCR)-confirmed SARS CoV-2 infection at the time of inclusion. Among other visits, the patients received either telephone or on-site follow-up examinations at three and 12 months (referred to as 3MFU and 12MFU, respectively) after their initial baseline visit. The choice between telephone and on-site follow-ups depended on the heath care level or consent to bio sample collection. Detailed information about the study was published elsewhere [17]. Only adults (age ≥ 18 years) who had at least a 3MFU were eligible for our analysis. A complete flow chart depicting sample selection and dropouts during analysis is shown in Supplementary Fig. S1. Our sample was recruited between December 2, 2020 and October 25, 2022. The last included 12MFU was carried out on May 31, 2023. Data were exported on June 15, 2023. All reporting adheres to the STROBE guidelines for cohort studies (Supplementary File S1).
Post-COVID syndrome score (PCS score) mapping and enhancement
The PCS score was calculated based on self-reported symptoms using a hypothesis-free clustering procedure that employed k-means clustering alongside other techniques. It consists of 12 subordinate sets of symptoms, including chemosensory deficits, fatigue, exercise intolerance, joint or muscle pain, ear-nose-throat ailments, coughing/wheezing, chest pain, gastrointestinal ailments, neurological ailments, dermatological ailments, infection signs, and sleep disturbance, as reported by Bahmer et al. [14]. If a symptom of the respective symptom complexes was present, the symptom indicators were multiplied by an individual point value ranging from two to seven representing its contribution to PCS severity. The assigned points were then summed up to a total score, which can be used to categorize the severity classes of PCS into distinct classes (none: 0 points; mild: > 0 to ≤ 10.75; moderate > 10.75 to ≤ 26.25; severe: > 26.25).
For mapping the PCS score in the SUEP, we identified 25 symptom items suitable to represent the 12 complexes. A list of included data items can be found in Table S1. In the SUEP, symptoms were assessed continuously and longitudinally during the course of the study. Symptom occurrence was further specified by start date and duration using categorical time intervals (e.g.“1 day”,“8–14 days”, “31–60 days (1–2 months)”). In the following analyses, we refer to the 3MFU, unless otherwise specified.
In order to meet the WHO definition of Post-COVID, symptoms must have persisted for at least two months prior to 3MFU [3]. Therefore, we calculated the time intervals between the onset of symptom and the visit date. We used the categorical duration intervals to determine a minimum and a maximum end date. A symptom complex was considered present if the start date was at least 61 days prior to the 3MFU date and the minimum end date was not earlier than the 3MFU date. To evaluate changes in the PCS score severity over time, we also calculated the PCS score during the 12MFU visit. In order to establish a clearer link between symptoms and infection, it was necessary for symptoms to persist for at least 11 months (335 days) and the “6–12 months “ duration category had to be selected. For symptoms labeled as “ongoing”, we used the date on which the corresponding template was last updated as the symptom end date.
After conducting the initial analysis, we discovered that some symptom complexes, e.g. fatigue, were underrepresented in comparison to existing literature [14, 18, 19]. To address this, we included additional elements from the following PROMs in our assessment: Chalder Fatigue Scale (CFS), PROMIS-29 Fatigue, PROMIS Dyspnea, PROMIS Cognitive Function, and PROMIS-29 Sleep disturbance (see Table S1). As suggested by Jackson [20], we recoded the CFS items to dichotomous items, created a sum score and categorized it as either “no fatigue” (sum score ≤ 3) or “fatigue” (sum score > 3). For PROMIS-29 Fatigue, PROMIS Dyspnea, PROMIS Cognitive Function and PROMIS-29 Sleep disturbance, we calculated sum scores and categorized these using T-Scores. T-Scores < 55 indicate no impairments for fatigue, dyspnea and sleep, while T-scores ≥ 55 indicate impairments, respectively [21]. The corresponding sum scores for fatigue [22], dyspnea [23], and sleep [24] were 11, 15, and 13, respectively. Cognitive impairments were indicated by a T-score ≤ 45, while a T-score ≥ 45 indicated no cognitive impairments [25]. The corresponding sum score was 15 [21]. If one item was missing or answered as “no information available”, a sum score could not be calculated for PROMIS-29 Fatigue, PROMIS Cognitive Function and PROMIS-29 Sleep Disturbance. To calculate a sum score for PROMIS Dyspnea, at least four items needed to be answered. Supplementary Text 1 details versions of the PROMs used in the SUEP. In the following analyses, we primarily used the mapping version that includes the PROM assessments, aligning with the fatigue prevalence reported in the literature [9, 13].
If a PROM sum score could not be calculated or the start date or duration of all symptoms per symptom complex was missing, the corresponding complex was marked as missing. If at least one complex was missing, no score was calculated.
Assessment of functional impairments and quality of life
To identify the effects and classify clinical relevance of PCS severity on physical and social impairments, we evaluated the PROMIS-29 “Ability to participate in social roles and activities” and “Physical function”. We categorized impairment and no impairment using a sum score cut-off of 13 and 18, respectively [26, 27]. QoL was measured using the European Quality of Life 5-Dimensions 5-Level Version (EQ-5D-5L) index and Visual Analogue Scale (VAS). The study assessed the health status of patients based on five dimensions: mobility, caring for oneself, everyday activities, pain, and anxiety. The patients rated each dimension on a five-point Likert-scale ranging from 1 (no problems) to 5 (inability/extreme problems). The scores were then calculated into an index score between 0 (worst condition) and 1 (optimal health) [28, 29]. Additionally, the patients reported their present health status on the VAS with 0 representing the worst health status and 100 the best health status.
Data analysis
Mean (M) and standard deviation (SD) were calculated for metric items, and absolute frequencies and percentages were reported for categorical and dichotomous items. Significance of group differences was assessed using the Chi-squared test or Fisher‘s exact test for categorical items, as appropriate. Due to the lack of a normal distribution for all continuous items (Shapiro–Wilk-Test), group differences were assessed using the Kruskal–Wallis-Test. Post-hoc tests were performed using Dunn’s-test. When appropriate, p values were Bonferroni-adjusted. Correlation analyses were performed using Spearman-correlation, with Spearman’s correlation coefficient rho considered to be small if |rho| > 0.1, medium if |rho| > 0.3 and large if |rho| > 0.5 [30]. All p values below 0.05 were considered statistically significant. We performed sensitivity analysis by treating missing symptom clusters as “not present”. R Version 4.2.3 was used for statistical analysis.
Results
Patient characteristics
The study sample comprised 854 patients, all of whom completed the 3MFU, from 27 study centers. The calculation of PCS score was possible for 581 patients (68.0%) without PROMs and for 603 patients (70.6%) with PROMs at 3MFU. Of those patients with PCS score including PROMs, 97.0% had a 12MFU (n = 585). The mean age of those patients with valid PCS score (including PROMs) was 54.0 years (SD = 16.1). Approximately 61% of patients (n = 365) were male. During the acute phase of COVID-19 infection, 81.6% of patients (n = 492) were hospitalized. Of these, 82.7% (n = 407) did not receive oxygen or received oxygen with less than 15 l/min (equal to WHO progression scale moderate). Forty-three percent of patients received at least one vaccination against SARS-CoV-2 prior to study inclusion (n = 240), of whom 45.4% (n = 179) received all reported vaccinations prior to study inclusion. Of the patients, 28.0% (n = 157) received two vaccinations, while 26.1% (n = 146) received three or more doses. The most frequently mentioned pre-existing comorbidities were cardiovascular diseases (n = 278, 46.1%), diabetes (n = 101, 16.8%), and cancer (n = 103, 17.1%). Twenty-nine percent had no comorbidities, while 70.7% of patients had at least one comorbidity. Please refer to Table 1 for further information.
Symptom complexes and Post-COVID syndrome score (PCS score)
For PCS score mapping, prior to PROM inclusion, exercise intolerance as demonstrated by shortness of breath was the most frequently reported symptom complex (20.3%), followed by neurological ailments (12.2%) and coughing/wheezing (10.4%). None of the patients reported sleep disturbances (in the free text field). After PROM inclusion, fatigue was most common (41.1%), followed by neurological ailments (39.4%) and exercise intolerance (39.7%). Sleep disturbances according to PROMs were present in 140 patients (16.4%). Detailed information is given in Table 2. The PCS score group allocation shifted from 62.0% (n = 360) to 35.7% (n = 215) for patients with no, 14.5% (n = 124) to 14.1% (n = 120) with mild, 14.1% (n = 82) to 36.7% (n = 221) with moderate and 2.6% (n = 15) to 7.8% (n = 47) with severe PCS after PROM integration (Fig. 1).
Presence of PCS at the 3MFU according to the PCS score by Bahmer et al. [14] with or without Patient Reported Outcome Measures (PROMs)
Differences between PCS score groups
When comparing PCS score groups, we found higher frequencies for female sex (p = 0.034*) and the presence of pre-existing respiratory diseases (p < 0.001*) (Table 1). Post-hoc tests showed that the comparisons between sex groups were only significant between no vs. severe (p < 0.001*), mild vs. severe (p = 0.039*) and moderate vs. severe PCS (p = 0.032*). QoL (measured using EQ-5D-5L index and VAS) differed significantly between PCS score groups at 3MFU and 12MFU (all p < 0.001*). Tables S2 to S4 display additional group comparisons and post-hoc tests. Stratified by sex and age, significant differences were observed between hospitalization and PCS score groups for female patients under 65 years of age (p = 0.035*) (Table S6). Post-hoc tests revealed significant differences between no vs. moderate (p = 0.013*), no vs. severe (p = 0.020*), and mild vs. severe PCS (p = 0.048*) (Table S7).
Correlation analyses showed a small but significant correlation between the PCS score and increasing BMI (p = 0.004*, rho = 0.121). This correlation was only present for females younger than 65 years (p = 0.001*, rho = 0.249) when stratified by sex and age. Similar effects were found for clinical severity at acute infection in general (p = 0.011*, rho = 0.104) and when stratified for female patients younger than 65 years (p = 0.002*, rho = 0.226). The number of pre-existing comorbidities increased as PCS score increased (p = 0.004*), but the effect size was small (rho = 0.116). When stratified by sex and age, the correlation was only significant for male patients (< 65 years: p = 0.006*, rho = 0.170; ≥ 65 years: p = 0.018*, rho = 0.229). The EQ-5D-5L index and VAS correlated significantly with increasing PCS score values at 3 and 12MFU (all p < 0.001*). This correlation had a strong negative effect (all rho ≤ -0.5) indicating a decreasing QoL with increasing PCS score (Table S8).
Changes in PCS severity from 3 to 12 month follow-up
Of the 603 patients who had a PCS score at 3MFU, 68.5% (n = 413) also had a PCS score at 12MFU. Among them, 12.1% experienced a worsening in PCS severity by one or two groups (Fig. 2). Of those, 60.3% were male with a mean age of 57.2 years (SD = 16.8). The most frequently reported pre-existing comorbidity (mean 2.0 (SD = 2.0) was cardiovascular disease, which was present in 52.1% of patients. In contrast, patients whose PCS score improved over time (14.1%, 63.5% male) were younger (mean age 49.9 years (SD = 14.6)) and had fewer comorbidities (mean 1.5 (SD = 1.7) (Additional information in Table S9). At the 12MFU, the most symptom complexes were fatigue, exercise intolerance (representing dyspnea) and neurological ailments (indicating cognitive impairments). These symptom complexes had similar relative frequencies as at the 3MFU for both PCS scores with and without PROMs (Table S10).
Functional aspects
At the 3MFU, 28.9% of patients (n = 160) exhibited social deprivations, while 41.9% (n = 236) had functional deficiencies. A group comparison between the PCS score and the selected PROMIS instruments revealed that only a few patients reported impairments without having a PCS according to the PCS score. Specifically, 6.7% (n = 8) of patients in the “no PCS” group had functional deficiencies according to PROMIS-29, and 1.7% (n = 2) reported social deprivation. Most of the patients’ impairments can be attributed to pre-existing comorbidities, primarily cardiovascular diseases (Table S11). For both instruments, the prevalence of impairments increased with PCS severity (Table 1). Correlation analysis also revealed a positive association between higher PCS score values and greater functional deficiencies (p < 0.001*, rho =−0.593) and social deprivation (p < 0.001*, rho = 0.635) (Table S8).
Sensitivity analysis
Not considering missing data in symptom complexes may affect the results. We assessed the potential impact of missing data in a symptom complex by assigning 0 points in the PCS score. Our analysis revealed no major differences in the overall results compared to the main analyses (Table S12).
Discussion
Post-COVID is a major public health concern [31] affecting both the individual’s QoL and society as a whole [15]. Applying the PCS score by Bahmer et al. in the cross-sectoral SUEP cohort of mostly hospitalized patients, our study showed differences in sex and QoL between PCS severity groups and significant correlations between PCS score and BMI, severity of acute infection, number of pre-existing comorbidities, and QoL. To adequately display the score in the SUEP cohort, we included PROMs in the score mapping. The calculation of the score identified 44.5% of patients with at least moderate PCS and 64.3% of patients with at least mild PCS outcome at 3MFU.
Other existing definitions, severity classification, and scoring systems for Post-COVID
Ongoing endeavors are directed at characterizing and quantifying PCS in cohort studies. These methodologies predominantly emphasize the assessment of symptoms, the QoL, and ability to perform daily activities [13,14,15,16, 32]. In addition to Bahmer et al.'s PCS score, there are other definitions with a different focus or distribution.
Derived from the National Institute of Health’s Researching COVID to Enhance Recovery (RECOVER) in the United States, Thaweethai et al. sought to diagnose and identify PCS [15]. Their proposed symptom score attempts to diagnose PCS through a scoring system for 12 symptoms, selected for their specificity to PCS and differentiation from other causes of symptoms. Additionally, a cutoff for PCS positivity is established. A ORCHESTRA study by Gentilotti et al. [13] identified four clinical phenotypes in PCS: respiratory, chronic fatigue-like, neurosensory, chronic pain. PCS severity was assessed using the SF-36 questionnaire, which revealed diverse risk factors and impacts on QoL depending on the phenotype. Gentilotti et al. suggest that the PCS severity can be quantified based on the number of present clusters. Clustering approaches suggest that PCS may consists of multiple phenotypes and underlying pathomechanisms [13, 18].
In contrast, the PCS score by Bahmer et al. [14] focuses primarily on the severity and classification of PCS symptoms (see Methods for more information), but is not primarily designed for diagnosis per se. Although this score was developed based on three sites and a large sample size, the study did not reach the number of neither sites, participants, nor geographic heterogeneity of the RECOVER and ORCHESTRA studies. However, it has the advantage of being developed in a relatively large population-based sample and being designed as a potential marker of progression and possible outcome in clinical trials. We introduced this score in the SUEP cohort to harmonize the definitions and research activities within the NAPKON consortium and to test the transferability in another scenario. We have successfully demonstrated the clinically meaningful applicability of the PCS score in our study and contributed to the confidence of the score as a severity and classification tool.
Selection of data items for score mapping and clinical applicability
In the POP study, Bahmer et al. did not initially include PROMs in the score development, arguing that “Asking the questions necessary to calculate the PCS score should take no longer than a few minutes and is therefore easy to implement in clinical practice.” [14]. However, transferring the score to other studies introduces new complexities that are closely connected to the type of symptom assessment used in each study. The use of PROMs has relevant drawbacks, including limited availability, constrained clinical applicability due to complexity, and practical barriers related to infrastructure and staff burden [33]. Nevertheless, PROMs also offer advantages such as confidence through validation, enhancing reliability compared to unstructured symptom inquiries, and objective measurements of patient perceptions. In our study, emphasizing the use of PROMs is essential to mitigate the potential underrepresentation of some symptoms (e.g., fatigue without PROMs 8.3%, with PROMs 41.1%) [9, 13] due to differences in the assessment methods and a potentially inadequate questioning. As demonstrated by this heterogeneous symptom prevalence, we highlight the importance of a standardized approach to symptom assessment, although this may reveal heterogeneous data quality. Consequently, in deriving implications and recommendations for other cohorts, it is crucial to consider that the PCS score will only be perceptible if either all relevant questions are already asked in some form or non-directly asked questions can be extracted from other parts of the dataset (e.g., PROMs). An initial evaluation of the frequency distribution of individual symptom complexes is important to assess the plausibility of approaches and ascertain whether fatigue might not be the predominant symptom.
First insights into the applicability of the PCS score and the quantification of PCS were demonstrated in a PCS outpatient clinic [34]. As the PCS score requires few resources (only one interview), it could be used at all levels of patient care and can be easily implemented in a trial if it is integrated from the beginning.
Clinical relevance, quality of life and functional assessments
Despite the varying methodologies adopted across different cohorts, there exists a consensus among most researchers and clinicians regarding the significance of evaluating PCS in conjunction with QoL and functional capacities [3]. They utilize diverse QoL measurements as correlates to arrive at an applicable and clinically meaningful PCS definition (e.g., PCS score: EQ-5D-5L, Gentilotti et al. definition: SF-36) [13, 14]. When applying the PCS score [14] to the SUEP cohort, we observed comparable correlations between QoL assessed by EQ-5D-5L, functional deficits, social participation, and the PCS severity.
Risk factors of PCS
Current research identifies female sex, increased age, pre-existing comorbidities, acute severity, and obesity as the most important determinants for PCS [35]. Our results align with this overall picture, as we identified inter-class differences for sex and pre-existing respiratory diseases. The correlation analysis revealed that higher BMI and a greater number of comorbidities were associated with more severe PCS. Although the role of psychiatric comorbidities was a major finding in the POP study, our investigation only revealed a tendency towards group differences that did not reach statistical significance. This observation may be attributed to disparities in the composition of the study population and the utilization of a smaller sample size. Although Bahmer et al. used other indicators of acute disease severity, such as number of symptoms, in a retrospective survey [14], we can confirm the correlation between acute severity based on the WHO progression scale, and higher PCS scores in the SUEP cohort. Especially the severity of the acute infection is a crucial factor in addressing PCS through either preventive intervention (e.g., vaccination), risk stratification or derived therapeutic decisions (e.g., medication for high-risk patient groups). Vaccination is a key protective factor for acute severity [36, 37], but its effect on PCS varies across studies [38]. Out study contributes to the ongoing debate by showing no differences in vaccination between PCS groups. However, this work did not investigate the effects of the type and timing of vaccinations in depth.
Prevalence estimates and differentiation from pre-existing conditions
Given the heterogeneous definitions and population characteristics, the prevalence of PCS varies accordingly. Current literature mostly suggests a prevalence of PCS of 10–20% [3, 39], but studies describe a considerably range and risk of bias for prevalence estimates from 2–51% depending on the population studied, the hospitalization status at acute infection, and the PCS (severity) definition [9, 15, 35]. Bahmer et al. described the prevalence of “severe” PCS in the population-based cohort as 13–20% [14]. In the SUEP cohort, only 7.8% of patients had a severe PCS outcome according to the score. However, we identified at least moderate PCS in 44.5% and at least mild PCS in 64.3% of patients at 3MFU. Overall, the aggregated numbers appear to be rather high but still are in line with the literature, depending on the stratification and sensitivity. The observed discrepancies may be due to both the hospitalization rate (SUEP cohort: 83.8%) and differences in study methodology and design [35, 40]. Specifically, the separation of “none “ and “mild “ PCS groups significantly shifts in prevalence. The selection of an appropriate threshold is related to the discussion of the clinical relevance and treatment requirements of individual symptoms and their effects on QoL and functional capacity.
The existing research on PCS is challenged to address several pre-existing conditions [35]. It is crucial to distinguish PCS from comorbidities and psychiatric disorders, as functional impairments can often be attributed to these conditions. A study that corrected for symptoms prior to COVID-19 infection identified approximately one in eight individuals in the general population as experiencing PCS symptoms [12]. However, the baseline value, which indicated whether functional impairments were pre-existing, is often unavailable. Therefore, data analysis requires adjustments to isolate the effects of comorbidities. We hypothesize that many studies do not sufficiently adjust for pre-existing conditions. Apart from checking the duration of symptoms according to the WHO definition, no further adjustment for comorbidities was performed in our analysis, but these complexities should be addressed in the aftermath. Additionally, there may be other potential biases or explanations for the described symptoms, such as the Post-Intensive Care syndrome [41]. In summary, it is possible that we have overestimated the prevalence of PCS in the SUEP cohort.
Strengths and limitations
The symptom mapping and methodology were discussed by a large interdisciplinary group of researchers. Furthermore, our analysis is based on a multi-center, prospective cohort study with in-depth assessments at 3 and 12 months after the acute infection. It is important to note that there are some limitations to our study, including missing baseline values for PROMs and the fact that the type of symptom assessment used in the SUEP cohort was not specifically designed for the targeted score. Approximately only 40% of the patients recruited in the SUEP were included in our analysis due to missing data at the 3MFU. An updated data export could potentially allow the analysis of more patients. The results indicate a low prevalence of high PCS among elderly patients (aged 65 years or older). Reasons for this may be that the respective patient group could not participate in the study or died before 3MFU, potentially limiting the generalizability of our results. Missing data were not handled using multiple imputation procedures. However, sensitivity analyses confirmed that there were no relevant differences between a complete case analysis and treating a missing symptom cluster as not present. This work focuses on the initial implementation of the PCS score in the SUEP cohort. Multivariable analyses were not aimed at in this study. Further analyses are needed to investigate the implications of risk factors and long-term outcomes of COVID-19.
Conclusion
In conclusion, this study emphasizes the importance of standardized definitions and further investigation of the clinical presentation of PCS. The study confirms the applicability of the PCS score across health care settings for severity stratification and highlights its association with severity in acute infection. Furthermore, we demonstrated that the score generates clinically meaningful correlates of PCS and reflects patients ‘ functional status and quality of life. Hence, the score appears to be adequate to quantify the impact of symptoms and PCS severity. However, the high prevalence and score thresholds require further discussion. These findings are crucial for designing targeted studies on PCS pathogenesis, selecting high-risk patients for inclusion in clinical trials and clinical decision-making. Additionally, considering the impact of functional activity on social participation and work ability is critical for a comprehensive assessment of PCS.
Materials/code availability
The code for PCS calculation will be made available in the epicodr31, a R-package developed by the Epidemiology Core Unit, an infrastructure of the NUM Clinical Epidemiology and Study Platform (NUKLEUS). The data are accessible through the NAPKON Use and Access procedure (https://napkon.de/use-and-access/).
Data availability
No datasets were generated or analysed during the current study.
References
Lemhöfer C, Best N, Gutenbrunner C, Loudovici-Krug D, Teixido L, Sturm C. Gefühlte und reale Arbeitsfähigkeit von Patient*innen mit Post-COVID Symptomatik nach mildem Akutverlauf: eine Analyse des Rehabilitation Needs Questionnaire (RehabNeQ). Phys Med Rehabil Kurortmed. 2022;32:151–8. https://doi.org/10.1055/a-1674-8044.
Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2022;94:253–62. https://doi.org/10.1002/jmv.27309.
World Health Organization. Post COVID-19 condition (Long COVID). https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition. Accessed 17 Aug 2023.
National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN) and Royal College of General Practitioners. Guideline COVID-19 rapid guideline: managing the long-term effects of COVID-19 2022.
Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed 16 Oct 2023.
Chaichana U, Man KKC, Chen A, Wong ICK, George J, Wilson P, et al. Definition of Post–COVID-19 condition among published research studies. JAMA Netw Open. 2023;6: e235856. https://doi.org/10.1001/jamanetworkopen.2023.5856.
Haslam A, Olivier T, Prasad V. The definition of long COVID used in interventional studies. Eur J Clin Invest. 2023; 00:e13989. https://doi.org/10.1111/eci.13989.
Lemhöfer C, Appel KS, Häuser W, Hettich N, Kohls M, Polidori MC. Post COVID – Just a matter of definition? Dtsch Med Wochenschr. 2022;147:1391–7. https://doi.org/10.1055/a-1940-1222.
O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2023;55: 101762. https://doi.org/10.1016/j.eclinm.2022.101762.
Morin CM, Bjorvatn B, Chung F, Holzinger B, Partinen M, Penzel T, et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med. 2021;87:38–45. https://doi.org/10.1016/j.sleep.2021.07.035.
Sepúlveda-Loyola W, Rodríguez-Sánchez I, Pérez-Rodríguez P, Ganz F, Torralba R, Oliveira DV, et al. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J Nutr Health Aging. 2020;24:938–47. https://doi.org/10.1007/s12603-020-1469-2.
Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–61. https://doi.org/10.1016/S0140-6736(22)01214-4.
Gentilotti E, Górska A, Tami A, Gusinow R, Mirandola M, Rodríguez Baño J, et al. Clinical phenotypes and quality of life to define post-COVID-19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine. 2023;62: 102107. https://doi.org/10.1016/j.eclinm.2023.102107.
Bahmer T, Borzikowsky C, Lieb W, Horn A, Krist L, Fricke J, et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: a prospective, multi-centre, population-based cohort study. EClinicalMedicine. 2022;51: 101549. https://doi.org/10.1016/j.eclinm.2022.101549.
Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329:1934–46. https://doi.org/10.1001/jama.2023.8823.
Klok FA, Boon GJAM, Barco S, Endres M, Geelhoed JJM, Knauss S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56:2001494. https://doi.org/10.1183/13993003.01494-2020.
Schons M, Pilgram L, Reese J-P, Stecher M, Anton G, Appel KS, et al. The German National Pandemic Cohort Network (NAPKON): rationale, study design and baseline characteristics. Eur J Epidemiol. 2022;37:849–70. https://doi.org/10.1007/s10654-022-00896-z.
Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38: 101019. https://doi.org/10.1016/j.eclinm.2021.101019.
Hartung TJ, Neumann C, Bahmer T, Chaplinskaya-Sobol I, Endres M, Geritz J, et al. Fatigue and cognitive impairment after COVID-19: a prospective multicentre study. EClinicalMedicine. 2022;53: 101651. https://doi.org/10.1016/j.eclinm.2022.101651.
Jackson C. The Chalder Fatigue Scale (CFQ 11). Occup Med (Lond). 2015;65:86. https://doi.org/10.1093/occmed/kqu168.
HealthMeasures. PROMIS® Score Cut Points. https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/promis-score-cut-points. Accessed 16 Oct 2023.
HealthMeasures. User Manual and Scoring Instructions - PROMIS Fatigue. https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manual_Only/PROMIS_Fatigue_User_Manual_and_Scoring_Instructions_02202023.pdf. Accessed 16 Oct 2023.
HealthMeasures. PROMIS Dyspnea Scoring Manual. https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manuals_/PROMIS_Dyspnea_Scoring_Manual.pdf. Accessed 16 Oct 2023.
HealthMeasures. PROMIS Sleep Scoring Manual. https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manual_Only/PROMIS_Sleep_Scoring_Manual_03June2022.pdf. Accessed 16 Oct 2023.
HealthMeasures. PROMIS Cognitive Function Scoring Manual. https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manual_Only/PROMIS_Cognitive_Function_Scoring_Manual_03June2022.pdf. Accessed 16 Oct 2023.
HealthMeasures. Ability to Participate in Social Roles and Activites Scoring Manual. https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manual_Only/PROMIS_Ability_to_Participate_in_Social_Roles_and_Activities_Scoring_Manual_03June2022.pdf. Accessed 16 Oct 2023.
HealthMeasures. User Manual and Scoring Instructions PROMIS Physical Function. https://www.healthmeasures.net/images/PROMIS/manuals/Scoring_Manual_Only/PROMIS_Physical_Function_User_Manual_and_Scoring_Instructions_02202023.pdf. Accessed 16 Oct 2023.
Nürnberger C, Kohls M, Rainers M, Miljukov O. epicodr: Primary Coding for NAPKON data; 2023. https://nukleus-ecu.github.io/epicodr/index.html. Accessed 16 Oct 2023.
Fraser M, Jagtar SN. eq5d: Methods for Analysing 'EQ-5D' Data and Calculating 'EQ-5D' Index Scores; 2023. https://cran.r-project.org/web/packages/eq5d/index.html. Accessed 16 Oct 2023.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: L. Erlbaum Associates; 1988.
Adeloye D, Elneima O, Daines L, Poinasamy K, Quint JK, Walker S, et al. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9:1467–78. https://doi.org/10.1016/S2213-2600(21)00286-1.
Sivan M, Preston N, Parkin A, Makower S, Gee J, Ross D, et al. The modified COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm) patient-reported outcome measure for Long Covid or Post-COVID-19 syndrome. J Med Virol. 2022;94:4253–64. https://doi.org/10.1002/jmv.27878.
Kyte DG, Calvert M, van der Wees PJ, ten Hove R, Tolan S, Hill JC. An introduction to patient-reported outcome measures (PROMs) in physiotherapy. Physiotherapy. 2015;101(2):119–25. https://doi.org/10.1016/j.physio.2014.11.003.
Lemhöfer C, Bahmer T, Baumbach P, Besteher B, Boekel A, Finke K, et al. Variations and predictors of Post-covid syndrome severity in patients attending a Post-COVID outpatient clinic. J Clin Med. 2023;12:4013. https://doi.org/10.3390/jcm12124013.
Nittas V, Gao M, West EA, Ballouz T, Menges D, Hanson SW, et al. Long COVID through a public health lens: an umbrella review. Public Health Rev. 2022;43:1604501. https://doi.org/10.3389/phrs.2022.1604501.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. E Engl J Med. 2020;383:2603–15. https://doi.org/10.1056/NEJMoa2034577.
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–85. https://doi.org/10.1056/NEJMoa2113017.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–46. https://doi.org/10.1038/s41579-022-00846-2.
Tabacof L, Nicolau E, Rivera A, Putrino D. Post-COVID conditions and burden of disease. Phys Med Rehabil Clin N Am. 2023;34(3):499–511. https://doi.org/10.1016/j.pmr.2023.04.007.
Zuin M, Mazzitelli M, Cattelan A. Long-COVID: is it time to revise the definition. J Med Virol. 2023;95: e29011. https://doi.org/10.1002/jmv.29011.
Vrettou CS, Mantziou V, Vassiliou AG, Orfanos SE, Kotanidou A, Dimopoulou I. Post-intensive care syndrome in survivors from critical illness including COVID-19 patients: a narrative review. Life. 2022;12(1):107. https://doi.org/10.3390/life12010107.
Acknowledgements
The study was carried out using the clinical-scientific infrastructure of NAPKON (Nationales Pandemie Kohorten Netz, German National Pandemic Cohort Network) and NUKLEUS (NUM Klinische Epidemiologie- und Studienplattform, NUM Clinical Epidemiology and Study Platform) of the Network of University Medicine (NUM), funded by the Federal Ministry of Education and Research (BMBF). The NUM funding reference number is 01KX2121.
We gratefully thank all NAPKON sites who contributed patient data and/or bio samples for this analysis. The representatives of NAPKON sites contributing at least 5 per mille to this analysis are (alphabetical order):Bielefeld University, Medical School and University Medical Center OWL, Bielefeld (Alsaad K, Hamelmann E, Heidenreich H, Hornberg C, Kulamadayil-Heidenreich NSA, Maasjosthusmann P, Muna A, Ruwe M, Stellbrink C), Helios Hospital Duisburg, Duisburg (Buechner N), Practice for general medicine Dashti, Eberswalde (Dashti Y, Tessmer C), Practice for general medicine Egestorf Bispingen, Bispingen (Laumerich B), Practice for general medicine Tennental, Deckenpfronn (Silberbaur I), University Hospital Augsburg, Augsburg (Bader S, Engelmann M, Fuchs A, Langer A, Maerkl B, Messmann H, Muzalyova A, Roemmele C), University Hospital Carl Gustav Carus, Dresden (Altmann H, Berner R, Dressen S, Koch T, Lindemann D, Seele K, Spieth P, Toepfner N, v. Bonin S), University Hospital Duesseldorf, Duesseldorf (Feldt T, Keitel V, Killer A, Knopp L, Luedde T, Lutterbeck M, Paluschinski M, Pereira JPV, Timm J), University Hospital Erlangen, Erlangen (Kraska D, Kremer AE, Leppkes M, Mang J, Neurath MF, Prokosch HU, Schmid J, Vetter M, Willam C, Wolf K), University Hospital Frankfurt, Frankfurt am Main (Arendt C, Bellinghausen C, Cremer S, Groh A, Gruenewaldt A, Khodamoradi Y, Klinsing S, Vehreschild M, Vogl T), University Hospital Hamburg-Eppendorf, Hamburg (Addo M, Almahfoud M, Engels ALF, Jarczak D, Kerinn M, Kluge S, Kobbe R, Petereit S, Schlesner C, Zeller T), University Hospital Leipzig, Leipzig (Baber R, Bercker S, Krug N, Mueller SD, Wirtz H), University Hospital Muenster, Muenster (Boeckel G, Meier JA, Nowacki T, Tepasse PR, Vollenberg R, Wilms C), University Hospital RWTH Aachen, Aachen (Dahl E, Marx N, Mueller-Wieland D, Wipperfuerth J), University Hospital Regensburg, Regensburg (Brochhausen-Delius C, Burkhardt R, Feustel M, Haag O, Hansch S, Malfertheiner M, Niedermair T, Schuster P, Wallner S), University Hospital Schleswig-Holstein, Kiel (Cleef S, Friedrichs A, Kaeding N, Koerner M, Kujat C, Oberlaender M, Pape D, Plagge M, Rupp J, Schunk D), University Hospital Schleswig-Holstein, Luebeck (Cleef S, Friedrichs A, Kaeding N, Koerner M, Kujat C, Oberlaender M, Pape D, Plagge M, Rupp J, Schunk D), University Hospital Technical University Munich, Munich (Barkey W, Erber J, Fricke L, Lieb J, Michler T, Mueller L, Schneider J, Spinner C, Winter C), University Hospital Tuebingen, Tuebingen (Bitzer M, Bunk S, Göpel S, Haeberle H, Kienzle K, Mahrhofer H, Malek N, Rosenberger P, Struemper C, Trauner F), University Hospital Wuerzburg, Wuerzburg (Frantz S, Frey A, Haas K, Haertel C, Herrmann J, Isberner N, Liese J, Meybohm P, Schmidt J, Schulze P), University Hospitals of the Ruhr University Bochum, Bochum (Brinkmann F, Brueggemann Y, Gambichler T, Hellwig K, Luecke T, Reinacher-Schick A, Schmidt WE, Schuette C, Steinmann E, Torres Reyes C), University Medical Center Goettingen, Goettingen Hafke A, Hermanns G, Nussbeck SY, Santibanez-Santana M, Zeh S), University Medicine Essen, Essen (Brochhagen L, Dolff S, Elsner C, Krawczyk A, Madel RJ, Otte M, Witzke O), University Medicine Greifswald, Greifswald (Becker K, Doerr M, Nauck M, Piasta N, Schaefer C, Schaefer E, Schattschneider M, Scheer C, Stahl D), University Medicine Oldenburg, Oldenburg (Arlt A, Griesinger F, Guenther U, Hamprecht A, Juergens K, Kluge A, Meinhardt C, Meinhardt K, Petersmann A, Prenzel R).
We gratefully thank all participating NAPKON infrastructures that contributed to this analysis. The representatives of these NAPKON infrastructures are (alphabetical order): University Hospital Cologne, Cologne (Brechtel M, Laugwitz M, Lee C, Sauer G, Schulze N, Seibel K, Stecher M), University Hospital Frankfurt, Frankfurt am Main (Hagen M, Schneider J, Sikdar S, Weismantel C, Wolf L), University Hospital Wuerzburg, Wuerzburg (Günther K, Haug J, Haug F,), University Hospital Wuerzburg and University of Wuerzburg, Wuerzburg (Fiessler C, Heuschmann PU, Schmidbauer L), University of Wuerzburg, Wuerzburg (Jiru-Hillmann S), University Medicine Greifswald, Greifswald (Bahls T, Hoffmann W, Nauck M, Schaefer C, Schattschneider M, Stahl D, Valentin H), University Medicine Goettingen, Goettingen (Chaplinskaya I, Hanß S, Krefting D, Pape C, Rainers M, Schoneberg A, Weinert N), Helmholtz Center Munich, Munich (Kraus M, Lorenz-Depiereux B), Charité—Universitaetsmedizin Berlin, Berlin (Lorbeer R, Schaller J, Fricke J, Krist L, Rönnefarth M, Schmidt S), University Hospital Schleswig-Holstein, Kiel (Bahmer T, Hermes A, Krawczak M, Lieb W, Schreiber S, Tamminga T).
We gratefully thank the NAPKON Steering Committee: University Hospital Giessen and Marburg, Giessen (Herold S), University of Wuerzburg, Wuerzburg (Heuschmann P), Charité—Universitaetsmedizin Berlin, Berlin (Heyder R), University Medicine Greifswald, Greifswald (Hoffmann W), Hannover Unified Biobank, Hannover Medical School, Hannover (Illig T), University Hospital Schleswig-Holstein, Kiel (Schreiber S), University Hospital Cologne and University Hospital Frankfurt, Charité—Universitaetsmedizin Berlin, Berlin (Witzenrath M).
K. Alsaad, E. Hamelmann, H. Heidenreich, C. Hornberg, N. S. A. Kulamadayil-Heidenreich, P. Maasjosthusmann, A. Muna, M. Ruwe, C. Stellbrink, N. Buechner, Y. Dashti, C. Tessmer, B. Laumerich, I. Silberbaur, S. Bader, M. Engelmann, A. Fuchs, A. Langer, B. Maerkl, H. Messmann, A. Muzalyova, C. Roemmele, H. Altmann, R. Berner, S. Dressen, T. Koch, D. Lindemann, K. Seele, P. Spieth, N. Toepfner, S. V. Bonin, T. Feldt, V. Keitel, A. Killer, L. Knopp, T. Luedde, M. Lutterbeck, M. Paluschinski, J. P. V. Pereira, J. Timm, D. Kraska, A. E. Kremer, M. Leppkes, J. Mang, M. F. Neurath, H. U. Prokosch, J. Schmid, M. Vetter, C. Willam, K. Wolf, C. Arendt, C. Bellinghausen, S. Cremer, A. Groh, A. Gruenewaldt, Y. Khodamoradi, S. Klinsing, M. Vehreschild, T. Vogl, M. Addo, M. Almahfoud, A. L. F. Engels, D. Jarczak, M. Kerinn, S. Kluge, R. Kobbe, S. Petereit, C. Schlesner, T. Zeller, R. Baber, S. Bercker, N. Krug, S. D. Mueller, H. Wirtz, G. Boeckel, J. A. Meier, T. Nowacki, P. R. Tepasse, R. Vollenberg, C. Wilms, E. Dahl, N. Marx, D. Mueller-Wieland, J. Wipperfuerth, C. Brochhausen-Delius, R. Burkhardt, M. Feustel, O. Haag, S. Hansch, M. Malfertheiner, T. Niedermair, P. Schuster, S. Wallner, S. Cleef, A. Friedrichs, N. Kaeding, M. Koerner, C. Kujat, M. Oberlaender, D. Pape, M. Plagge, J. Rupp, D. Schunk, S. Cleef, A. Friedrichs, N. Kaeding, M. Koerner, C. Kujat, M. Oberlaender, D. Pape, M. Plagge, J. Rupp, D. Schunk, W. Barkey, J. Erber, L. Fricke, J. Lieb, T. Michler, L. Mueller, J. Schneider, C. Spinner, C. Winter, M. Bitzer, S. Bunk, S. Göpel, H. Haeberle, K. Kienzle, H. Mahrhofer, N. Malek, P. Rosenberger, C. Struemper, F. Trauner, S. Frantz, A. Frey, K. Haas, C. Haertel, J. Herrmann, N. Isberner, J. Liese, P. Meybohm, J. Schmidt, P. Schulze, F. Brinkmann, Y. Brueggemann, T. Gambichler, K. Hellwig, T. Luecke, A. Reinacher-Schick, W. E. Schmidt, C. Schuette, E. Steinmann, C. Torres Reyes, A. Hafke, G. Hermanns, S. Y. Nussbeck, M. Santibanez-Santana, S. Zeh, L. Brochhagen, S. Dolff, C. Elsner, A. Krawczyk, R. J. Madel, M. Otte, O. Witzke, K. Becker, M. Doerr, M. Nauck, N. Piasta, C. Schaefer, E. Schaefer, M. Schattschneider, C. Scheer, D. Stahl, A. Arlt, F. Griesinger, U. Guenther, A. Hamprecht, K. Juergens, A. Kluge, C. Meinhardt, K. Meinhardt, A. Petersmann, R. Prenzel, M. Brechtel, M. Laugwitz, C. Lee, G. Sauer, N. Schulze, K. Seibel, M. Stecher, M. Hagen, J. Schneider, S. Sikdar, C. Weismantel, L. Wolf, K. Günther, J. Haug, F. Haug, C. Fiessler, P. U. Heuschmann, L. Schmidbauer, S. Jiru-Hillmann, T. Bahls, W. Hoffmann, M. Nauck, C. Schaefer, M. Schattschneider, D. Stahl, H. Valentin, I. Chaplinskaya, S. Hanß, D. Krefting, C. Pape, M. Rainers, A. Schoneberg, N. Weinert M. Kraus, B. Lorenz-Depiereux, R. Lorbeer, J. Schaller, J. Fricke, L. Krist, M. Rönnefarth, S. Schmidt, T. Bahmer, A. Hermes, M. Krawczak, W. Lieb, S. Schreiber, T. Tamminga, S. Herold, P. Heuschmann, R. Heyder, W. Hoffmann, T. Illig, S. Schreiber, M. Witzenrath
Funding
Open Access funding enabled and organized by Projekt DEAL. This publication was supported by the German Federal Ministry of Education and Research (BMBF) Network of University Medicine 2.0: “NUM 2.0" [Grant No. 01KX2121], Project: National Pandemic Cohort Network (NAPKON).The project National Pandemic Cohort Network (NAPKON) is part of the Network of University Medicine (NUM), funded by the German Federal Ministry of Education and Research (BMBF) (FKZ: 01KX2121). Parts of the infrastructure of the Würzburg study site were supported by the Bavarian Ministry of Research and Art to support Corona research projects. Parts of the NAPKON project suite and study protocols of the Cross-Sectoral Platform are based on projects funded by the German Center for Infection Research (DZIF).
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: C. Lemhöfer, K. S. Appel, C. Nürnberger, M. Kohls, C. M. Polidori; Methodology: C. Lemhöfer, K. S. Appel, C. Nürnberger, T. Bahmer, M. Kohls; Formal analysis and investigation: C. Nürnberger, K. S. Appel, M. Kohls; Writing—original draft preparation: K. S. Appel, C. Nürnberger; Writing—review and editing: C. Lemhöfer, T. Bahmer, I. Bröhl, K. Fiedler, C. Förster, R. Geisler, T. Kraus, J. Petersen, C. M. Polidori, G. Rohde, M. Scherer, P. Wagner, J. P. Reese, M. Kohls, J. Butzmann; N. Hettich-Damm, S. Blaschke, H. Dashti, J. Deckert, M. Dreher, C. Finke, F. Hanses, S. M. Hopff, B. E. O. Jensen, M. Konik, K. Lehnert, S. M. Nunes de Miranda, L. Mitrov, O. Miljukov, K. Tausche, J. J. Tebbe, JJ. Vehreschild, F. Voit, M. Weigl; Supervision: C. Lemhöfer.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. For the NAPKON SUEP, a primary ethics vote was obtained at the Ethics Committee of the Department of Medicine at Goethe University Frankfurt (local ethics ID approval 20–924). All further study sites received their local ethics votes at the respective ethics commissions. The NAPKON SUEP is registered at ClinicalTrials.gov (Identifier: NCT04768998). Approval for this study was granted by the Ethics Committee of Friedrich-Schiller-University Jena, Germany (2023–3029-Daten, 26.06.2023).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
TB received funding from Network of University Medicine (NUM)/ German Federal Ministry of Education and Research (BMBF)—COVID-19 related research grants for the conduct of the different NAPKON platforms, including the NAPKON-POP platform that the COVIDOM study is based in. NAPKON support code is 01KX2021.German Center for Lung Research (DZL)—Unrestricted Research Grants for the conduct of research activities related to Asthma and Allergy, AstraZeneca, GlaxoSmithKline (consulting fees), AstraZeneca, GlaxoSmithKline, Novartis, Roche, Chiesi, Boeringer-Ingelheim, Merck, Pfizer (payment or honoraria for lectures), Chiesi, AstraZeneca (support for attending meetings and/or travel), CoVit-2 (NCT04751604) (participation on a Data Safety Monitoring Board or Advisory Board). MCP received royalties for the publication of two books: the latest edition of ‘Paziente Anziano—Paziente Geriatrico—Medicina della Complessita’, EdiSES 2020 and ‘Ratgeber ` Altern—Es ist nie zu spat’, Elsevier 2021. MCP reveived funding from Consultant memodio, multimodale App für MCI (Mild Cognitive Impairment) (consulting fee), Scientific Board Member: National Guideline on Comprehensive Geriatric Assessment (AWMF S3-Guideline) and co-chair of the special interest group CGA of the EuGMS (European Geriatric Medicine Society), Editorial Board Member of Aging Research Reviews und ‘Deutsche Medizinische Wochenschrift’. JD received funding from University Hospital Wuerzburg (institutionell) (support for the present manuscript and support for travel), German Federal Ministry of Education and Research (BMBF) (grants or contracts), Max-Planck-Institute of Psychiatry (Participation on a Data Safety Monitoring Board or Advisory Board), Network of University Medicine (NUM)/ German Federal Ministry of Education and Research (BMBF)- Co-Speaker Mental Health Group, Deutsche Gesellschaft für Biologische Psychiatrie (German Society of Biological Psychiatry (DGBP))—President and Treasurer, Deutsche Gesellschaft für Psychiatrie, Psychotherapie, Psychosomatik und Nervenheilkunde (German Society of Psychiatry, Psychotherapy, Psychosomatic Medicine and Neuropsychiatry (DGPPN))—Section Speaker. MD received funding from Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen (Ministry of Culture and Science of the State of North Rhine-Westphalia)—Contract “Beyond COVID-19 “, Gilead – advisory Bard (consulting fees), Gilead, GSK and Astra Zeneca (payment or honoraria for lectures). CF reveived funding from Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, Grant numbers FI 2309/1–1 (Heisenberg Program) FI 2309/2–1 and 327654276 (SFB 1315)), German Ministry of Education and Research (BMBF Grant numbers 01GM1908D, 01GM2208C, 13GW0566D, 01GM2102, 01EP2201). BEOJ received funding from GSK, Gilead Sciences, ViiV, MSD (consulting fees), GSK, Gilead Sciences, ViiV, Pfizer, AstraZeneca, Janssen-Cilag, Fresenius Medical Care and Falk Foundation (payment or honoraria for lectures), Gilead Sciences (support for attending meetings and/or travel. FH received funding from Bavarian State Ministry of Health and Care (Bayrisches Staatsministerium für Gesundheit und Pflege)—Post-COVID outpatient treatment center (contract), Academy for Infectious Medicine, Deutsche Gesellschaft Interdisziplinäre Notfall- und Akutmedizin (DGINA) e.V.and bavarian Landesaerztekammer (payment or honoraria for lectures). BEOJ received funding from GSK, Gilead Sciences, ViiV, MSD (consulting fees), GSK, Gilead Sciences, ViiV, Pfizer, AstraZeneca, Janssen-Cilag, Fresenius Medical Care and Falk Foundation (Payment or honoraria for lectures), Gilead Sciences (support for attending meetings and/or travel). JPR received funding from Landesaerztekammer Hessen (payment or honoraria for lectures), German Ministry of Health (BMG) (expert testimony), Grants or contracts from German Ministry of Research and Education (within NUM, CAEHR, RECAP), Bavarian State (ministry for science and the arts—DigiOnko), Federal Joint Committee (G-BA) within the Innovationfond (Peri-OP, StaerkeR), German Center for Lung Research (within PASSION). GR received personal fees from Astra Zeneca, Atriva, Boehringer Ingelheim, GSK, Insmed, MSD, Sanofi, Novartis and Pfizer for consultancy during advisory board meetings, also personal fees from Astra Zeneca, Berlin Chemie, BMS, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Sanofi, Solvay, Takeda, Novartis, Pfizer and Vertex for lectures including service on speakers bureaus. JJV received funding from Merck / MSD, Gilead, Pfizer, Astellas Pharma, Basilea, German Centre for Infection Research (DZIF), University Hospital Freiburg/ Congress and Communication, Academy for Infectious Medicine, University Manchester, German Society for Infectious Diseases (DGI), Ärztekammer Nordrhein, University Hospital Aachen, Back Bay Strategies, German Society for Internal Medicine (DGIM), Shionogi, Molecular Health, Netzwerk Universitätsmedizin, Janssen, NordForsk, Biontech, APOGEPHA, German Federal Ministry of Education and Research (BMBF), Deutsches Zetrum für Luft- und Raumfahrt (DLR), University of Bristol, Rigshospitalet Copenhagen. FV received funding from Gilead Sciences, MSD, B.Braun Melsungen (grants or contracts), Gilead Sciences (payment or honoraria for lectures), Gilead Sciences and Viiv Healthcare (support for attending meetings and/or travel). MW is board member of German Society of Physical and Rehabilitation Medicine (Deutsche Gesellschaft für Physikalische und Rehabilitative Medizin e.V. (DGPRM), unpaid), Network of University Medicine (NUM)/ German Federal Ministry of Education and Research (BMBF)—Speaker Physical and Rehabilitation Group (unpaid). CL reveived institutional funding from Federal Joint Committee (G-BA) within the Innovationfond (WATCH, Grant numbers L01NVF22114), Editorial Board. Member of ‘Physikalische Medizin, Rehabilitationsmedizin, Kurortmedizin’, Network of University Medicine (NUM)/ German Federal Ministry of Education and Research (BMBF)—Speaker Physical and Rehabilitation Group (unpaid), board member of German Society of Physical and Rehabilitation Medicine (Deutsche Gesellschaft für Physikalische und Rehabilitative Medizin e.V. (DGPRM), unpaid). All other authors reported no conflicts of interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Appel, K.S., Nürnberger, C., Bahmer, T. et al. Definition of the Post-COVID syndrome using a symptom-based Post-COVID score in a prospective, multi-center, cross-sectoral cohort of the German National Pandemic Cohort Network (NAPKON). Infection (2024). https://doi.org/10.1007/s15010-024-02226-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02226-9