Abstract
Pollution of the environment by microscopic fragments of plastic is a growing environmental concern. A category of this pollution is fiber fragments from textiles, a source of which is believed to be fibers released by clothing made of synthetic fibers during laundering. These fragments could enter the environment at the end of the textile’s useful life if it is not re-used or recycled. Disposal of biodegradable fibers could be achieved by industrial composting, but natural fibers are sometimes modified during manufacturing in ways that might influence biodegradation. The composting behavior of fabrics made with unmodified and chemically modified wool fibers (chlorine-Hercosett treated), regenerated cellulose, and several synthetic fibers was compared in industrial composting conditions according to an established standard test method. The fabrics were characterized by Fourier transform infrared spectroscopy, energy dispersive X-ray and electron microscopy. The regenerated cellulose (viscose rayon) biodegraded to the greatest extent in the test, and both types of wool also biodegraded readily. All three synthetic fibers had no biodegradation. The machine-washable wool biodegraded more rapidly than unmodified wool and analysis of residues at the conclusion of the test indicated that it did not generate non-degradable fiber fragments. The epicuticle of unmodified wool is covered with a hydrophobic layer, which may resist microbial attack, but with time this slowly degraded. Conversely, the machine-washable wool is hydrophilic and therefore was easier for microbes to attack. If not re-used, commercial, machine-washable wool textiles can be readily disposed of in industrial composting conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollution of the environment by microscopic fragments of plastic is a growing envi ronmental concern throughout the world (Gavigan et al. 2020; Browne et al. 2011), and a large proportion of these materials are in the form of textile fibers (Sanchez-Vidal et al. 2018), released by textiles during their lifetime, for example during laundering (Napper and Thompson 2016). However, the fate of textiles at the end of their useful life, if they aren’t re-used or recycled, is also an issue, which is only increasing with the advent of ‘fast fashion’ (Bick et al. 2018). They may end up in landfill where they may persist or break down extremely slowly, but in theory textiles constructed entirely from natural fibers such as cotton or wool, or from regenerated biopolymers, such as viscose rayon, could be disposed of through industrial composting facilities. However, commercially-produced textiles, including those made from natural fibers, contain synthetic components in the form of colorants and finishes that improve their performance and aesthetic appeal. If composting is to be a routine disposal method for natural fiber textiles, then the influence on biodegradation behavior of chemical finishes including synthetic polymeric finishes used on the fiber needs to be understood. The behavior of textile fibers during composting is also somewhat informative to their biodegradation in lower intensity aerobic conditions, such as shallow soil burial.
Wool is a natural fiber comprised of keratin protein and therefore is expected to be biodegradable. It is used in a wide variety of textile applications from activewear, formal wear such as suits, through to interior textiles such as carpets and upholstery, and technical applications (Johnson et al. 2003). Wool to be used in apparel applications often has a chemical treatment carried out on it to make it machine-washable. This is done by a two-step process of chemical modification by a strong oxidation treatment that damages the wool fiber scales and makes the fiber surface hydrophilic by removing the hydrophobic 18-methyl eicosanoic acid (18-MEA) layer bonded to the epicuticle of the wool fiber surface by a thioester linkage. This allows binding of a very thin layer of a hydrophilic polyamide resin (e.g. Hercosett) film onto it (Hassan and Carr 2019). The treatment prevents the wool fibers from being able to ‘felt’ together; felting being an outcome of the interaction of the wool fiber’s natural surface scales (Schofield 1938). The film covers the fibers preventing felting shrinkage. The presence of this polyamide film on the surface of the fiber could conceivably retard the fiber’s biodegradation behavior, and if not biodegradable itself, might be a source of microplastic fragments. This would potentially compromise wool’s natural and sustainable credentials.
The rapid biodegradation behavior of wool is well known for certain terrestrial environments and it has been informally demonstrated to biodegrade readily when buried in the soil (The Campaign for Wool 2014; Innovation in Textiles 2012). The process of biodegradation is thought to initially involve cleavage of disulfide bridges (responsible for much of the mechanical robustness of wool), followed by hydrolytic degradation (Szostak-Kotowa 2004). Broda et al. studied the biodegradation of wool geotextiles in the form of coarse ropes and found that they survived for about one vegetation season (Broda et al. 2016), which was slower than cellulosic materials and therefore more beneficial for the establishment of plants. McNeil et al. demonstrated ‘closed loop’ recycling of wool carpet via soil burial and as well as demonstrating wool’s biodegradability, the work showed that it acted as a fertilizer, increasing the plant growth compared to a control plot without wool applied (McNeil et al. 2007). In the earliest work that examined the influence of chemical treatments on wool, Hodgson et al. studied biodegradation in natural topsoil of commercial wool apparel fabrics with different dyes and finishes (Hodgson et al. 2023). In that work the rate of biodegradation varied according to the treatment, but all were ultimately biodegradable. The machine-washable wool fabric included in that trial biodegraded slightly slower than untreated wool. Synthetic fabrics were included in that study and showed no biodegradation. Another study showed that wool and cotton fabrics biodegraded in soil more rapidly than bio-based poly (lactic acid) fiber (Sun et al. 2013). Anselmi et al. demonstrated that recycled wool fibers, recovered from reprocessed wool textiles biodegraded readily in aquatic conditions (Anselmi et al. 2023) and these fibers may have had a range of unknown chemical treatments applied. Nevertheless, other than the single soil burial study described above (Hodgson et al. 2023), no published literature reported the effect of commonly used shrink-resist treatments on wool’s biodegradation behavior. Moreover, there do not appear to have been studies that examine the biodegradation behavior of commercial wool fabrics in comparison with other fiber types in industrial composting situations.
The work reported here describes an investigation into the composting biodegradation behavior of commercial wool fabrics that had different finishes applied to them, and compared them with equivalent synthetic fibers. A standard method for measuring biodegradation of materials under laboratory conditions was used (International Standards Organisation 2012). An advantage of this approach over soil burial is that it uses a strictly controlled environment (i.e. a bioreactor) compared to the natural biodegradation environments used in previous work, which allows for accurate quantification of the rate of biodegradation and the potential to examine residues that have low levels of non-target material. The experimental work was undertaken between September 2019 and June 2020, at Lincoln, Canterbury, New Zealand.
Materials and methods
Fabrics
Five lightweight, single-jersey knitted apparel fabrics were sourced by The Woolmark Company (Shanghai, China), constructed from untreated wool, machine-washable wool (chlorine-Hercosett treated), polyester, polyamide (nylon) and polypropylene. These fabrics were manufactured to be as close as possible to the same specification, and all are intended for the same application, i.e. as a ‘next-to-skin’ or base layer. This means that they differed only in the type of fiber from which they were constructed. These are typical of the types of fabrics used in garments that consumers have multiple items of, are worn frequently and are replaced relatively often. All fabrics were finished commercially including being dyed to a pale-blue shade, with the exception of the polypropylene. An additional fabric made from viscose rayon (regenerated cellulose) was included as a natural fiber comparison. This was a lightweight woven fabric (sourced locally, dyed to a beige color), so it was different in structure to the other five, but as discussed below, the fabrics were deconstructed before testing to minimize fabric structure effects. Thus, there were a total of six fabrics included in the biodegradation trial. Fabric details are provided in Table 1. The nominal carbon content is provided as this is the basis on which the sample size is determined for the biodegradation test.
Fabric preparation
Sufficient fabric was taken from the bulk quantities and subjected to a washing process to simulate them being part way into their lifecycle. All fabrics except the untreated wool were subjected to two International Standardization Organization (ISO) ‘5A’ standard washes in a Wascator laboratory washing machine, except the untreated wool, which received two ISO ‘7A’ washes (the 5A cycles would have severely felted this fabric; the 7A cycle is gentler so more appropriate for a fabric that would require hand-washing) (Woolmark 2016). These simulate the mechanical degradation of approximately 20 domestic washes (Smith 1990), and two further 7A cycles were added for all fabrics, to simulate additional detergent damage that could be present this far into a product’s lifetime. The detergent used was the standard SDCE ECE Phosphate Reference Detergent (B) (SDC Enterprises 2021).
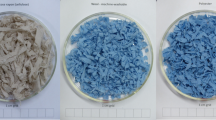
These fabric specimens were then deconstructed, to present them all in an equivalent physical form for the biodegradation test, i.e. to minimize the influence of any fabric structure effects. The fabrics were shredded in a heavy-duty paper shredder. Shredding was carried out twice on each fabric sample, with the second shredding being perpendicular to the first, resulting in small pieces of fabric with dimensions in the order of millimeters to centimeters. The deconstructed samples also included yarn fragments and some loose fiber. The shredder was thoroughly cleaned before use and between each specimen, so any cross-contamination would be at levels low enough to not influence the outcomes of biodegradation testing. By way of example, images of three of the shredded fabrics are shown in Fig. 1.
Biodegradation method
The biodegradation test was carried out according to ISO 14855-1-2012: Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions (International Standards Organisation 2012). The principle of the test is to measure the CO2 evolved by the samples as they biodegrade, i.e., as the material’s carbon (natural or synthetic fiber polymer in this situation) is converted into CO2 by decomposition. The test method allows for the option of carrying out the trial using compost as the medium, or vermiculite that has been inoculated with compost microbes. Vermiculite is a porous, biologically inert mineral material (Schulze 2005) and the advantage of its use is that during the biodegradation trial only the test specimen releases substantial CO2. When compost is used, there is a CO2 contribution from the biodegradation of the compost itself. This would be the same for all materials under test, but by removing it from the system a higher level of accuracy of CO2 measurement is possible. For this reason, vermiculite was chosen for this trial. In the test, the prepared fabric samples were mixed with vermiculite that had been inoculated with microbes extracted from compost as described in the standard test method. Three replicates of each material were tested, and the test was carried out with the vessels in a water bath at 58 ± 2 °C as specified in the method. This is a typical temperature encountered in industrial composting (Epstein 2011, Bioplastics 2009). The position of the vessels in the water bath was randomized. As well as the positive control, three ‘blanks’ with just the vermiculite and no sample were included. Oxygen and moisture levels were maintained above 6% and 60% respectively, and the reactor vessels (3 L glass jars) were shaken weekly. A specimen containing 20 g of carbon was placed in each vessel. The test was run for 181 days.
Selection of control material
The standard method used for this study (see previous section) specifies just one positive control material to be used: thin-layer chromatography grade cellulose (microcrystalline cellulose). This material presented some problems as a control for textile biodegradation in seawater in an earlier study (Collie et al. 2019) because it failed to stay well suspended in the seawater inoculum. However, in this study the biodegradation medium is solid material, so the need to ensure good suspension in liquid is removed; thus, the methodology was not modified in this regard. The viscose rayon fabric (cellulose) acted as an additional positive control in fiber/textile form.
Analysis methods
Residues from all fabrics were examined using a Hitachi scanning electron microscope (SEM; Model: TM 3030 Plus, Hitachi Corporation, Tokyo, Japan) without any conductive coating. The presence of a polyamide film on the surface of the machine-washable wool raises the potential concern that the biodegradation of this wool might release polyamide microplastic fragments into the environment. In order to investigate the potential for this to occur, the surface of the fibers before and after the degradation test was examined by a Perkin-Elmer Fourier-Transform Infrared (FTIR) spectroscope (Model: System 2000, Perkin Elmer Corporation, USA) and Energy-Dispersive X-ray spectroscopy (EDX; using the same SEM equipped with a Quantax75 energy dispersive X-ray attachment).
Results and discussion
Biodegradation behavior
The extent of biodegradation of each sample (conversion of carbon in the material to CO2 as measured by the respirometry system) after 181 days is provided in Table 2. The average of three replicates is provided along with the 95% confidence interval, and the relative biodegradation, i.e. the biodegradation of each as a percentage of that shown by the positive control is also provided. According to the standard test method (International Standards Organisation 2012), there are certain requirements relating to the behavior of the positive control in order for it to be a valid test for certification purposes. In this work the test was simply used as a research tool, for comparative purposes rather than for product accreditation, so those requirements were not directly relevant. However, they do give an indication of how well the test system performed overall so it is worthwhile to consider those performance levels. There are three criteria: a low between-replicate variability for the positive control (under 20% difference between maximum and minimum levels of biodegradation); a target range for CO2 formation level in the blank control after 10 days (50 to 150 mg CO2/g); a threshold biodegradation level for the positive control after 45 days (over 70%). Here the first two were easily achieved (2.2% and 92.2 mg/g respectively), but the third criterion was not quite met (66.4%). Nevertheless, by the end of the test the positive control showed a high level of biodegradation, i.e. 88% of the cellulose material had converted into CO2, so conditions were obviously very suitable for biodegradation to occur.
Considering the relative biodegradation of the test specimens, the highest relative biodegradation of the wool fabrics was shown by the machine-washable variant (~ 77%), followed by the untreated wool (~ 55%). The viscose rayon fabric (cellulose) was almost as biodegradable as the positive control (also cellulose), having a relative biodegradation of ~ 94%. All synthetic fibers showed virtually no CO2 production, indicating that they did not biodegrade at all under these conditions.
It appears that the treatment used in the machine-washable finish has an effect of increasing the overall degradability of the fiber, rather than decreasing it as might have been expected. This is likely to be because the chemical pre-treatment step has degraded the fiber’s scale surface and removed the hydrophobic 18-MEA layer, making the surface hydrophilic (Hassan and Leighs 2017), and more amenable to microbial attack causing a rapid biodegradation. Any effect from the polyamide film has not compensated for this. This film is hydrophilic, and swells in the presence of water (Hassan and Carr 2019), in which state it may present a substantially reduced barrier to microbial attack. Although the specimens are not immersed in water in this test, there are substantial amounts of moisture present (60% as required by the standard method), so the polyamide film is likely to be in a somewhat swollen state allowing the microbes to penetrate through the film. The untreated wool possesses an intact hydrophobic cuticle so has greater resistance to microbial degradation. Note that this result differs from that seen in a study that examined soil burial biodegradation of wool fabrics, where the machine-washable finish slowed biodegradation (Hodgson et al. 2023). In their work, however, moisture levels were much lower than in our study (in the range of 12 to 24% compared to ~ 60%), meaning that the film was not swollen and thus resisted infiltration by microbes.
The progression of biodegradation of the six fabrics studied up to 181 days is shown in Fig. 2, using the net cumulative CO2 production. This net value is calculated by subtracting the average cumulative CO2 production of the vermiculite-only replicates from the cumulative CO2 production for each fabric-plus-vermiculite replicate. This means that for fabrics with very low levels of biodegradation negative values can result, as is the case here for two of the polyester replicates.
In general, the behavior of the three replicates is consistent for each fiber type (as also indicated by the small confidence intervals in Table 2), although the machine-washable wool fabric had one replicate that showed slightly lower biodegradation than the other two. Observations of the very early stages of the test were that biodegradation for the control, viscose rayon and machine-washable wool started virtually immediately, but the untreated wool sample took several days to begin. This is probably due to the robust intact cuticle present on untreated wool (Caven et al. 2022) providing some initial resistance to biodegradation. The degradation of machine-washable wool was rapid in the first 20 days of the test and then slightly slowed. On the other hand, viscose rayon rapidly biodegraded in the first 50 days and then the degradation slowed down as most of the degradation occurred within the 50 days.
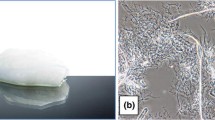
Images of the biodegradation medium at the completion of the test (vermiculite with fabric residues; one of the three replicates) for each specimen are provided in Fig. 3. The blank control (vermiculite only) and the positive control (microcrystalline cellulose) are also shown. The more highly degraded samples have an overall color much closer to that of the vermiculite itself, indicating they have diminished substantially in quantity, and have lost some of their color. The synthetic fibers (middle row) retained their original colors after the degradation test (noting that the polypropylene was undyed). On the other hand, untreated and machine-washable wool fibers almost completely lost their color, as observed by others in soil burial biodegradation (Hodgson et al. 2023), and the viscose rayon fabric became almost unrecognizable.
It is worthwhile to reflect on the biodegradation level of wool, sitting as it does between regenerated non-native cellulose materials (the positive control and fiber in the form of viscose rayon) and synthetic materials. In its native form wool has evolved to be relatively resistant to microbial attack—as needed to fulfil its biological function of protecting the animal on which it grows. It is made up of a robust structural protein (keratin) and has a structure that provides further resilience via a protective cuticle which includes bound lipids that impart hydrophobicity (Feldtman et al. 1983). Regenerated cellulose materials lack any similar protective layer, so it is to be expected that they will biodegrade readily. Conversely, synthetic polymers did not evolve to be a part of any natural ecosystem, so have inherently high biological resistance. The fact that wool degrades readily in composting conditions, soil burial (Broda et al. 2016; Hodgson et al. 2023) and in aqueous conditions (Sun et al. 2013; Anselmi et al. 2023) indicates that it strikes a happy medium: durable in use, but nonpersistent in the environment at the end of its useful life.
Scanning electron microscopy
Figures 4, 5 are representative SEM images of the two wool types, along with viscose rayon and polyester. These are provided here as they are of most interest considering the biodegradation results. The other synthetics are excluded as, like the polyester, they show virtually no change from the original state, consistent with their lack of biodegradation.
For both the untreated wool and machine-washable wool (Fig. 4), the low magnification images show a surprising level of fabric structure remaining after the biodegradation test, with knitted loops and yarns still visible. Under higher magnification, degradation of the fibers is more apparent, with fibers appearing almost ‘collapsed’, having some areas of exposed cortex and separated cortical cells. Nevertheless, there are still regions of reasonably intact cuticle (examples indicated by ovals). This level of biodegradation occurred within a 181-day test period, so it is expected that they would fully degrade within a short timeframe.
The presence of some apparently intact material at the macroscopic level after the biodegradation trial is probably an outcome of the ‘support’ provided by the vermiculite during biodegradation (e.g. during the periodic shaking that the vessels receive during the trial), meaning that they are perhaps less likely to physically disintegrate as they biodegrade. These samples are highly converted from material carbon to CO2 (the biodegradation level is 48% and 68% for untreated and machine-washable respectively), which shows that different parts of the sample degrade at different rates, rather than the whole material degrading at the same rate.
The micrographs for viscose rayon (Fig. 5) also show some intact textile structure, although it is less defined than for the wool samples. This fabric is woven (compared to knitted for the wool fabrics), and therefore is structurally more robust than the knitted wool fabrics. However, it is hard to discern a woven-like structure remaining, although yarn fragments seem to be visible. There are some very fine filaments apparent (shown in the image on the right), which are probably below one micrometer in size, suggesting that viscose rayon fiber fibrillates as it biodegrades. In Fig. 5 it can be seen that the polyester fabric and fibers show no apparent degradation, with just a few fragments of vermiculite (a larger piece is visible in the low magnification image). Overall, these images show fiber damage that corresponds to the level of biodegradation (Table 2).
Analysis of composting medium
Consistent with the standard test method, the contents of each biodegradation vessel was analyzed at the conclusion of the trial. The pH, moisture content, total volatile solids and carbon–nitrogen ratio were measured. None of these showed any pattern that related to differences in biodegradation behavior. The pH in the jars for all samples was the same on average, with values across all replicates in the range 7.4 to 8.3. Moisture content across all replicates was in the range of 64.7 to 76.2%, with no differences between fiber types. There were also no meaningful differences between fiber types for the other parameters analyzed.
The moisture content in the jars is sufficient that the hydrophilic polyamide film on the surface of the machine-washable wool fibers would likely be in a swollen state during biodegradation (Hassan and Carr 2019). As such it potentially provides a diminished barrier to microbial attack than it would if dry.
Analysis of fabric residues
In undertaking this analysis, the ability to distinguish between wool and polyamide (in the form of the Hercosett resin on the machine-washable wool fibers) is confounded by the qualitative similarity in their elemental composition: both proteins (i.e. wool) and polyamides are rich in amide groups. A key difference is the presence of sulfur in wool, which is not present in polyamide. FTIR was used with the goal of distinguishing differences in the presence of amide peaks in the fiber residues. With EDX the aim was to look at the elemental composition of the surfaces of residues, and as this is done in the SEM it was possible to see the specific target of the analysis, i.e. a region on a fiber or a microscopic particle.
Fourier-transform infrared spectroscopy (FTIR)
The fate of the polyamide film applied to the machine-washable wool fabric was investigated. Three spectra for wool fabrics are shown in Fig. 6, top. These are the biodegraded residues of untreated and machine-washable wool, plus non-degraded wool for comparison. Both biodegraded residues have spectra consistent with that of the non-degraded wool. The characteristic peaks of the non-degraded wool are all present in the two biodegraded samples. The IR spectrum of control wool fabric before the biodegradation test shows IR bands of wool, such as amide I, amide II and amide III bands at 1650, 1540, and 1236 cm−1 respectively, which is consistent with published results (Hassan and McLaughlin 2018). The spectrum of degraded unmodified wool fabric shows exactly the same IR bands although the intensity of the bands increased for the degraded unmodified wool. However, the intensity of hydroxyl band at 3100–3700 cm−1 increased highly but became sharp, which suggests that water molecules attached to the carboxyl groups produced due to the degradation of wool are causing an increase in the intensity of the IR band at this region. The spectrum of machine-washable wool also shows the same IR bands suggesting no presence of Hercosett resin in the degraded wool, indicating its degradation.
The spectra of nylon, polyester and polypropylene (Fig. 6, bottom) show typical IR bands of these polymers and the spectral database identified them as the same polymers. No formation of hydroxyl band for polypropylene and polyester confirms no degradation of these fibers. On the other hand, nylon fabric shows an IR band at 3300 cm−1, which also could be attributed to the hydroxyl groups of water molecules attached to the carboxyl groups of nylon by hydrogen bonding. The viscose fabric (Fig. 6, bottom) shows a broad IR band which became sharp at 3400 cm−1 suggesting high degradation of viscose rayon which produced carboxylic acids and this IR band is associated with the hydroxyl groups of water molecules attached to these carboxyl groups.
Energy dispersive X-ray spectroscopy (EDX)
As noted previously, the fate of the polyamide film used in making wool machine-washable is of particular interest. The untreated and machine-washable wool before and after composting, plus non-fibrous debris from the degraded machine-washable wool residues were examined. The latter was examined to determine if there were fragments having composition consistent with polyamide rather than wool, as these could conceivably be microplastic fragments. As was apparent from the SEM images (Sect. ”Scanning electron microscopy”), the residues were relatively intact and there were few candidate particles in the residue that could conceivably be microplastic fragments. Nevertheless, some fragmentary debris was analyzed along with the visibly intact fibers. These results are presented in Table 3. The C, N, O and S content of wool is 50.18, 19.87, 26.24 and 3.71, which is consistent with elemental composition of wool previously reported (Hassan 2019, Hassan 2021). The decrease in S content and increase in O content suggest the formation of hydroxyl and carboxyl groups due to the degradation of macromolecular chains of unmodified wool fibers.
Sulfur content is the most important element in this analysis as it is present in wool but not in polyamide, so would not be present in solid residues of the polyamide resin. It can be used to distinguish between wool and non-wool residues. It is apparent that for the non-degraded samples the machine-washable wool has reduced levels of sulfur on its surface compared to untreated wool (1.95% vs. 3.71%). This is because the layer of polyamide film (sulfur-free) is partially obscuring the wool surface underneath. Comparing the machine-washable wool before and after composting (intact fibers, i.e. 1.95% vs. 2.11%), there are very similar levels, implying that these intact fiber residues are similar in composition to non-degraded wool. The biodegraded machine-washable wool fragment material has a sulfur level of 1.75%, slightly lower than that for the intact fiber but the presence of sulfur still strongly supports the hypothesis that these fragments are wool fiber fragments and not polyamide film (Fig. 7). If it was the latter, it would be free of sulfur. This debris appears to be fragments of wool material in the process of biodegrading, and thus not microplastic fragments.
Conclusion
This biodegradation test carried out in composting conditions has shown the relative biodegradability fabrics of wool and other fiber types. From most biodegradable to least was viscose rayon > machine-washable wool > untreated wool > nylon = polyester = polypropylene. Machine-washable wool biodegraded readily because the chemical treatment used to introduce this attribute has had the effect of increasing the fiber’s vulnerability to microbial attack, rather than decreasing it as might have been expected. Untreated wool biodegraded more slowly, but still to a far greater extent than all the synthetic fibers, which were completely intact at the completion of the test. Viscose rayon biodegraded slightly faster than machine-washable wool.
SEM images of the fabric residues showed intact fragments of fabric and yarns present at the completion of the test, even when more than two-thirds of the specimen had been converted into CO2. Nevertheless, under higher magnification it was clear that these materials were thoroughly degraded.
Several analyses were undertaken to observe changes to the chemical characteristics of fibers after undergoing biodegradation. FTIR analysis did not enable any conclusions to be drawn as to the fate of the polyamide film (part of the treatment to make wool machine-washable) after biodegradation, but EDX analysis confirmed that smaller fragmentary debris present in the machine-washable wool sample did contain sulfur, and so was wool and not microplastic polyamide. Thus, it appears that the biodegradation of machine-washable wool under composting conditions does not create microplastic pollution.
This work has increased understanding of the biodegradation behavior of wool by establishing its behavior in composting conditions, adding to the existing knowledge for soil burial and marine biodegradation. Wool is durable during use but degrades readily in all of these situations, reducing its environmental impact both during its lifetime (by not being a source of persistent microfiber pollution) and after disposal (by biodegrading in a wide range of conditions, including industrial composting). A notable gap remaining in the knowledge of wool’s biodegradation behavior relative to other fibers is how it behaves under anaerobic conditions, as might be encountered in a landfill situation.
References
Anselmi S, Provenza F, Bentivoglio T, Picerno G, Cavallo A, Renzi M (2023) Marine biodegradability and ecotoxicity of MWool recycled wool fibers: a circular-economy-based material. Oceans 4:114–131
Bick R, Halsey E, Ekenga CC (2018) The global environmental injustice of fast fashion. Environ Health A Global Access Sci Sour 17:1–4
Bioplastics E (2009) Fact Sheet—Indusrial Composting. In: E.V., E. B. (ed). Berlin, Germany.
Broda J, Przybyło S, Kobiela-Mendrek K, Biniaś D, Rom M, Grzybowska-Pietras J, Laszczak R (2016) Biodegradation of sheep wool geotextiles. Int Biodeterior Biodegrad 115:31–38
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol 45:9175–9179
Caven B, Brook D, Taylor M, Connell S, Brown A (2022) Reverse engineering of a wool fibre to mimic the structural hierarchy of a gecko’s foot. Bioinspir Biomim 17:046018
Collie SR, Ranford S, Fowler I & Brorens P (2019) 4A3_0151_ Microfibre Pollution—What's the Story for Wool? Proceedings of the 19th World Textile Conference-Autex 2019, Ghent.
Epstein E (2011) Industrial composting. Environmental engineering and facilities management. New York: Taylor and Francis Group.
Feldtman HD, Leeder JD & Rippon JA (1983) Role of fibre structure in wool fibre and fabric performance. In: Postle RKS, Niwa M (eds) Objective evaluation of apparel fabrics. Japan: The Textile Machinery Society of Japan.
Gavigan J, Kefela T, Macadam-Somer I, Suh S, Geyer R (2020) Synthetic microfiber emissions to land rival those to waterbodies and are growing. PLoS ONE 15:e0237839
Hassan MM (2019) Enhanced colour, hydrophobicity, UV radiation absorption and antistatic properties of wool fabric multi-functionalised with silver nanoparticles. Colloids Surfaces A Physicochem Eng Aspects 581:123819
Hassan MM (2021) Energy-efficient dyeing of wool fabrics with sulfonic acid derivatives of aniline by oxidation polymerization. Sustain Mater Technol 29:e00290
Hassan MM, Carr CM (2019) A review of the sustainable methods in imparting shrink resistance to wool fabrics. J Adv Res 18:39–60
Hassan MM, Leighs SJ (2017) Effect of surface treatments on physicomechanical, stain-resist, and UV protection properties of wool fabrics. Appl Surf Sci 419:348–356
Hassan MM, McLaughlin JR (2018) Multi-functional wool fabrics by graft-copolymerisation with polystyrene sulphonate: their enhanced fire retardancy, mechanical properties, and stain-resistance. New J Chem 42:18919–18927
Hodgson A, Leighs SJ, Van Koten C (2023) Compostability of wool textiles by soil burial. Text Res J 93:15–16
Innovation in Textiles (2012) Research shows merino fabrics biodegrade rapidly [Online]. Available: https://www.innovationintextiles.com/research-shows-merino-fabrics-biodegrade-rapidly/ [Accessed 30th March 2021].
International Standards Organisation (2012) ISO 14855-1 Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions—Method of analysis by evolved carbon dioxide.
Johnson NAG, Wood EJ, Ingham PE, McNeil SJ, McFarlane ID (2003) Wool as a technical fibre. J Text Inst 94:26–41
McNeil SJ, Sunderland MR, Zaitseva LI (2007) Closed-loop wool carpet recycling. Resour Conserv Recycl 51:220–224
Napper IE, Thompson RC (2016) Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar Pollut Bull 112:39–45
Sanchez-Vidal A, Thompson RC, Canals M, de Haan WP (2018) The imprint of microfibres in southern European deep seas. PLoS ONE 13:e0207033
Schofield J (1938) 21—Researches on wool felting. Journal of the Textile Institute Transactions 29:T239–T252
Schulze DG (2005) Clay minerals. In: HILLEL, D. (ed.) Encyclopedia of Soils in the Environment. Oxford: Elsevier
SDC Enterprises (2021). ECE Phosphate Reference Detergent 'B' [Online]. Available: https://www.sdcenterprises.co.uk/products/sdce-ece-b-phosphate-sdce-type-3/ [Accessed 22nd March 2024].
Smith PJ (1990) Dimensional stability in domestic laundering. 8th International Wool Textile Research Conference. Christchurch, New Zealand.
Sun Y, Luo J, Ni AQ, Bi YY, Yu WD (2013) Study on biodegradability of wool and PLA fibers in natural soil and aqueous medium. Adv Mater Res 641–642:82–86
Szostak-Kotowa J (2004) Biodeterioration of textiles. Int Biodeterior Biodegrad 53:165–170
The campaign for wool (2014) Biodegradable Wool Test Complete! [Online]. Available: http://www.campaignforwool.org/2014/10/14/biodegradable-wool-test-complete/ [Accessed 20th March 2021].
Woolmark (2016) TM31 Dimensional stability. Woomark Specification AK-1.
Acknowledgements
We are grateful to Steve Ranford and Andy Cooper for their valuable discussions and assistance with reviewing this work.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by Australian Wool Innovation, contract number 4500012232.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Editorial responsibility: S.Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collie, S., Brorens, P., Hassan, M. et al. Biodegradation behavior of wool and other textile fibers in aerobic composting conditions. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05802-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05802-6