Abstract
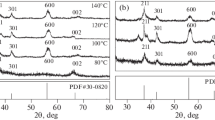
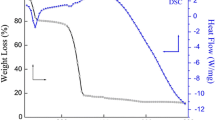
Zinc oxide (ZnO) nanoparticles with diverse morphologies were synthesized using an economical and environmentally friendly co-precipitation method. The samples underwent characterization through X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy, and energy-dispersive spectra analysis. The photocatalytic degradation capabilities of the synthesized samples were assessed using organic dyes and antibiotics as model pollutants. SEM images revealed nanospheres, nanorods, and nanotubes structures of ZnO under three distinct conditions. The average crystallite sizes of hexagonal wurtzite ZnO samples were determined using Debye–Scherrer’s formula from the full width at half maximum of XRD peaks, resulting in sizes of 52.80, 57.23, and 78.02 nm for ZnO nanospheres (ZnO-S), ZnO nanotubes (ZnO-T), and ZnO nanorods (ZnO-R), respectively. The optical band gap energies were measured at 3.25, 3.26, and 3.28 eV for ZnO-S, ZnO-T, and ZnO-R, respectively, demonstrating lower values compared to bulk ZnO (3.37 eV). Photocatalytic efficiency assessment using methylene blue indicated that ZnO-S exhibited the highest degradation efficiency (98.64%), followed by ZnO-T (97.63%) and ZnO-R (59.94%) under optimized conditions of 0.05 g/L catalyst dose, 90-min irradiation time, 130-W light intensity, and 1-mg/L methylene blue concentration. ZnO-S demonstrated effective photocatalysis in the ofloxacin degradation with a rate constant of 0.0112 min−1. According to a study on photocatalytic mechanisms, hydroxyl radicals play an important role in the degradation of pollutants. The photocatalyst maintains high photocatalytic efficiency even after five uses. Collectively, ZnO with different morphologies holds promise as a potential catalyst for environmental protection.















Similar content being viewed by others
Data availability
The data will be available on request.
Code availability
Not applicable.
References
Abdulrahman AF, Ahmed SM, Barzinjy AA, Hamad SM, Ahmed NM, Almessiere MA (2021) Fabrication and characterization of high-quality UV photodetectors based ZnO nanorods using traditional and modified chemical bath deposition methods. Nanomater 11:677. https://doi.org/10.3390/nano11030677
Ahmad K, Ghatak HR, Ahuja SM (2020) A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: a sustainable approach. Environ Technol Innovation 19:100893. https://doi.org/10.1016/j.eti.2020.100893
Ahmadi A, Hajilou M, Zavari S, Yaghmaei S (2023) comparative review on adsorption and photocatalytic degradation of classified dyes with metal/non-metal-based modification of graphitic carbon nitride nanocomposites: synthesis, mechanism, and affecting parameters. J Cleaner Prod 382:134967. https://doi.org/10.1016/j.jclepro.2022.134967
Akir S, Barras A, Coffinier Y, Bououdina M, Boukherroub R, Omrani AD (2016) Eco-friendly synthesis of ZnO nanoparticles with different morphologies and their visible light photocatalytic performance for the degradation of Rhodamine B. Ceram Int 42:10259–10265. https://doi.org/10.1016/j.ceramint.2016.03.153
Aldeen TS, Mohamed HEA, Maaza M (2022) ZnO nanoparticles prepared via a green synthesis approach: physical properties, photocatalytic and antibacterial activity. J Phys Chem Solids 160:110313. https://doi.org/10.1016/j.jpcs.2021.110313
Alkaykh S, Mbarek A, Ali-Shattle EE (2020) Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 6:4. https://doi.org/10.1016/j.heliyon.2020.e03663
Al-Mamun R, Rokon ZI, Rahim A, Hossain I, Islam S, Ali R, Bacchu S, Waizumi H, Komeda T, Khan ZH (2023) Enhanced photocatalytic activity of Cu and Ni-doped ZnO nanostructures: a comparative study of methyl orange dye degradation in aqueous solution. Heliyon 9:e16506. https://doi.org/10.1016/j.heliyon.2023.e16506
Altaf CT, Colak TO, Rostas AM, Popa A, Toloman D, Suciu M, Sankir ND, Sankir M (2023) Impact on the photocatalytic dye degradation of morphology and annealing-induced defects in zinc oxide nanostructures. ACS Omega 8:14952–14964. https://doi.org/10.1021/acsomega.2c07412
Baig N, Kammakakam I, Falatha W (2021) Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2:1821–1871. https://doi.org/10.1039/d0ma00807a
Behnajady MA, Tohidi Y (2015) The effect of operational parameters in the photocatalytic activity of synthesized Mg/ZnO–SnO2 nanoparticles. Desalin Water Treat 53:1335–1341. https://doi.org/10.1080/19443994.2013.852482
Brillas E (2020) A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 250:126198. https://doi.org/10.1016/j.chemosphere.2020.126198
Castro-Lopes S, Guerra Y, Silva-Sousa A, Oliveira DM, Gonçalves LAP, Franco A, Padron-Hern E, Pena-Garcia R (2020) Influence of pH on the structural and magnetic properties of Fe-doped ZnO nanoparticles synthesized by sol gel method. Solid State Sci 109:106438. https://doi.org/10.1016/j.solidstatesciences.2020.106438
Chae KW, Zhang O, Kim JS, Jeong YH, Cao G (2010) Low-temperature solution growth of ZnO nanotube arrays. Beilstein J Nanotechnol 1:128–134. https://doi.org/10.3762/bjnano.1.15
Chaisorn W, Nuengmatcha P, Noypha A, Pimsen R, Porrawatkul P, Kuyyogsuy A, Thepchuay Y, Sricharoen P, Limchoowong N, Chanthai S, Nuengmatcha P (2023) Adsorption-photocatalytic degradation abilities of γ-irradiated chitosan-ZnO-AgNP composite for organic dye removal and antibacterial activity. Environ Sci Pollut Res 30:96840–96859. https://doi.org/10.1007/s11356-023-29305-y
Chankhanittha T, Somaudon V, Watcharakitti J, Nanan S (2021) Solar light-driven photocatalyst based on bismuth molybdate (Bi4MoO9) for detoxification of anionic azo dyes in wastewater. J Mater Sci Mater Electron 32(2):1977–1991. https://doi.org/10.1007/s10854-020-04965-5
Chitra M, Mangamma G, Uthayarani K, Neelakandeswari N, Girija EK (2020) Band gap engineering in ZnO based nanocomposites. Physica E 119:113969. https://doi.org/10.1016/j.physe.2020.113969
Donkadokula NY, Kola AK, Saroj NI, D, (2020) A review on advanced physico-chemical and biological textile dye wastewater treatment techniques. Rev Environ Sci Biotechnol 19:543–560. https://doi.org/10.1007/s11157-020-09543-z
El-Moslamy SH, Elnouby MS, Rezk AH, El-Fakharany EM (2023) Scaling-up strategies for controllable biosynthetic ZnO NPs using cell free-extract of endophytic Streptomyces albus: characterization, statistical optimization, and biomedical activities evaluation. Sci Rep 13:3200. https://doi.org/10.1038/s41598-023-29757-9
El-Sharkawy HM, Shawky AM, Elshypany R, Selim H (2023) Efficient photocatalytic degradation of organic pollutants over TiO2 nanoparticles modified with nitrogen and MoS2 under visible light irradiation. Sci Rep 13:8845. https://doi.org/10.1038/s41598-023-35265-7
Esfahani EB, Dixit F, Zeidabadi FA, Johnson MR, Mayilswamy N, Kandasubramanian B, Mohseni M (2023) Ion exchange and advanced oxidation/reduction processes for per- and polyfluoroalkyl substances treatment: a mini-review. Curr Opin Chem Eng 42:100953. https://doi.org/10.1016/j.coche.2023.100953
Ge MY, Wua HP, Niua L, Liua JF, Chenb SY, Shenc PY, Zenga YW, Wanga YW, Zhanga GQ, Jiang JZ (2007) Nanostructured ZnO: from monodisperse nanoparticles to nanorods. J Cryst Growth 305:162–166. https://doi.org/10.1016/j.jcrysgro.2007.03.016
Giasari AS, Muharam APM, Syampurwadi A, Diana D, Eddy R, Primadona I (2023) Morphological effect of one-dimensional ZnO nanostructures on the photocatalytic activity. J Phys Chem Solids 176:111259. https://doi.org/10.1016/j.jpcs.2023.111259
Groeneveld I, Kanelli M, Ariese F, Bommel MR (2023) Parameters that affect the photodegradation of dyes and pigments in solution and on substrate—An overview. Dyes Pigm 210:110999. https://doi.org/10.1016/j.dyepig.2022.110999
Hanif MA, Kim YS, Akter J, Kim HG, Kwac LK (2023) Fabrication of robust and stable N-Doped ZnO/Single-walled carbon nanotubes: characterization, photocatalytic application, kinetics, degradation products, and toxicity analysis. ACS Omega 8(18):16174–16185. https://doi.org/10.1021/acsomega.3c00370
He X, Yang Y, Li Y, Chen J, Yang C, Liu R, Xu Z (2022) Effects of structure and surface properties on the performance of ZnO towards photocatalytic degradation of methylene blue. Appl Surf Sci 599:153898. https://doi.org/10.1016/j.apsusc.2022.153898
Hendrix Y, Rauwel E, Nagpal K, Haddad R, Estephan E, Boissière C, Rauwel P (2023) Revealing the dependency of dye adsorption and photocatalytic activity of ZnO nanoparticles on their morphology and defect states. Nanomaterials 13(13):1998. https://doi.org/10.3390/nano13131998
Hsu CY, Rheima AM, Sabri Abbas Z, Faryad MU, Kadhim MM, Altimari US, Dawood AH, Abed ZT, Radhi RS, Jaber AS, Hachim SK (2023) Nanowires properties and applications: a review study. S Afr J Chem Eng 46:286–311. https://doi.org/10.1016/j.sajce.2023.08.006
Hu X, Zhang Y, Zhang J, Yang H, Wang F, Fei B, Noor N (2022) Sonochemically-coated transparent wood with ZnO: passive radiative cooling materials for energy saving applications. Renewable Energy 193:398–406. https://doi.org/10.1016/j.renene.2022.05.008
Intharaksa O, Nanan S, Patdhanagul N, Panphojan T, Srikakul T, Tantisuwichwong N, Tantisuwichwong N, Dulya sucharit R (2023) Preparation of magnetic CuO/Fe3O4/ZnO photocatalyst for complete degradation of methylene blue under natural sunlight irradiation. J Phys Chem Solids 182:111577. https://doi.org/10.1016/j.jpcs.2023.111577
Kalhorizadeh T, Dahrazma B, Zarghami R, Mirzababaei S, Kirillov AM, Abazari R (2022) Quick removal of metronidazole from aqueous solutions using metal–organic frameworks. New J Chem 46:9440. https://doi.org/10.1039/d1nj06107k
Kanmani SS, Ramachandran K, Umapathy S (2012) Eosin yellowish dye-sensitized ZnO nanostructure-based solar cells employing solid PEO redox couple electrolyte. Int J Photoenergy 2012:267824. https://doi.org/10.1155/2012/267824
Kaur K et al (2020) Photodegradation of organic pollutants using heterojunctions: a review. J Environ Chem Eng 8:103666. https://doi.org/10.1016/j.jece.2020.103666
Khatun M, Mitra P, Mukherjee S (2023) Effect of band gap and particle size on photocatalytic degradation of NiSnO3 nanopowder for some conventional organic dyes. Hybrid Adv 4:100079. https://doi.org/10.1016/j.hybadv.2023.100079
Kotkar SN, Prasad S, Gadekar GP, Rewatkar SB (2022) Auto combustion synthesis of ZnO for degradation of organic dye under natural solar light with bactericidal activity. Inorg Chem Commun 144:109830. https://doi.org/10.1016/j.inoche.2022.109830
Kulik K, Lenart-Boroń A, Wyrzykowska K (2023) Impact of antibiotic pollution on the bacterial population within surface water with special focus on mountain rivers. Water 15:975. https://doi.org/10.3390/w15050975
Kumar DR, Ranjith KS, Haldorai Y, Kandasami A, Kumar TRR (2019) Nitrogen-implanted ZnO nanorod arrays for visible light photocatalytic degradation of a pharmaceutical drug acetaminophen. ACS Omega 4:11973–11979. https://doi.org/10.1021/acsomega.9b00557
Kumar V, Kumar P, Deka R, Abbas Z, Mobin SM (2022) Recent development of morphology-controlled hybrid nanomaterials for triboelectric nanogenerator: a review. Chem Rec 22(9):e202200067. https://doi.org/10.1002/tcr.202200067
Kumari C, Sharma P, Katyal SC, Tanwar M, Bamola P, Sharma H, Kumar R, Chhoker S (2022) Photocatalytic activity of GeSbSeEr quaternary chalcogenide for efficient methylene blue degradation in visible light. Results Surf Interfaces 9:100088. https://doi.org/10.1016/j.rsurfi.2022.100088
Kweinor TE, Rathilal S, Chetty M, Armah EK, Asante-Sackey D (2019) Treatment of water and wastewater for reuse and energy generation-emerging technologies. Water Wastewater Treatment 25:1–21. https://doi.org/10.5772/intechopen.84474
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innovation 3(2):275–290. https://doi.org/10.1016/j.biori.2019.09.001
Lemessa F, Simane B, Seyoum A, Gebresenbet G (2023) Assessment of the impact of industrial wastewater on the water quality of rivers around the Bole Lemi industrial park (BLIP), Ethiopia. Sustainability 15:4290. https://doi.org/10.3390/su15054290
Liu S, Bu Y, Cheng S, Tao R (2023) Synthesis of TiO2/g-C3N5 heterojunction for photocatalytic degradation of methylene blue wastewater under visible light irradiation: Mechanism analysis. Diamond Relat Mater 136:110062. https://doi.org/10.1016/j.diamond.2023.110062
Mahajan A, Sankar V, Ramaprabhu S, Nagar R (2022) Template-free microwave-assisted growth of 3D hexagonal ZnO rods. Mater Sci Eng, B 284:115901. https://doi.org/10.1016/j.mseb.2022.115901
Mishra PK, Dash A, Sen S (2020) Optical properties and defects study in Zn1-x (CrGa)x/2O. Mater Today: Proc 33:5687–5690. https://doi.org/10.1016/j.matpr.2020.04.213
Mishra S, Chakinala N, Chakinala AG, Surolia PK (2022) Photocatalytic degradation of methylene blue using monometallic and bimetallic Bi-Fe doped TiO2. Catal Commun 171:106518. https://doi.org/10.1016/j.catcom.2022.106518
Mohammed N, Palaniandy P, Shaik F (2021) Pollutants removal from saline water by solar photocatalysis: a review of experimental and theoretical approaches. Int J Environ Anal Chem 103:4155–4175. https://doi.org/10.1080/03067319.2021.1924160
Mustapha S, Ndamitso MM, Abdulkareem AS, Tijani JO, Shuaib DT, Mohammed AK, Sumaila A (2019) Comparative study of crystallite size using Williamson Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv Nat Sci: Nanosci Nanotechnol 10:045013. https://doi.org/10.1088/2043-6254/ab52f7
Nayak H, Padhi B (2023) Degradation of methylene blue using Ca-doped LaMnO3 as a photocatalyst under visible light irradiation. Results Chem 6:101104. https://doi.org/10.1016/j.rechem.2023.101104
Nithyaa K, Kalyanasundharam S (2019) Effect of chemically synthesis compared to biosynthesized ZnO nanoparticles using aqueous extract of C. halicacabum and their antibacterial activity. OpenNano 4:1000. https://doi.org/10.1016/j.onano.2018.10.001
Nuengmatcha P, Porrawatkul P, Chanthai S, Sricharoen P, Limchoowong N (2019) Enhanced photocatalytic degradation of methylene blue using Fe2O3/graphene/CuO nanocomposites under visible light. J Environ Chem Eng 7(6):103438. https://doi.org/10.1016/j.jece.2019.103438
Pan Y, Abazari R, Tahir B, Sanati S, Zheng Y, Tahir M, Gao J (2024) Iron-based metal–organic frameworks and their derived materials for photocatalytic and photoelectrocatalytic reactions. Coord Chem Rev 499:215538. https://doi.org/10.1016/j.ccr.2023.215538
Pinoargote-Chang MH, Fernández-Andrade A, Zambrano-Intriago LA, Quiroz-Fernández LS, Villanueva-Ramos G, Montenegro MC, Rodríguez-Díaz JM (2022) Photo-Fenton process for the degradation of blue 1 dye and estradiol benzoate hormone in binary system: application of sunlight and UV-C radiation. Case Stud Chem Environ Eng 6:100226. https://doi.org/10.1016/j.cscee.2022.100226
Plessis AD (2022) Persistent degradation: global water quality challenges and required actions. One Earth 5:2022. https://doi.org/10.1016/j.oneear.2022.01.005
Quadros SD, Metz DCHP, Zimmermann LM (2023) Efficient degradation of cationic dyes by ZnO and ZnO–Fe(III) quantum dots under natural sunlight and UV light. J Phys Chem Solids 18:111464. https://doi.org/10.1016/j.jpcs.2023.111464
Rajamanickam D, Shanthi M (2016) Photocatalytic degradation of an organic pollutant by zinc oxide—solar process. Arab J Chem 9:S1858–S1868. https://doi.org/10.1016/j.arabjc.2012.05.006
Rajput RB, Shaikh R, Sawant J, Kale RB (2022) Recent developments in ZnO-based heterostructures as photoelectrocatalysts for wastewater treatment: a review. Environ Adv 9:100264. https://doi.org/10.1016/j.envadv.2022.100264
Rattanaburi P, Nuengmatcha P, Pimsen R, Porrawatkul P (2023) Photocatalytic degradation of organic dyes on magnetically separable barium hexaferrite as photocatalyst under conditions of visible light irradiation. Environ Sci Pollut Res 30:68969–68986. https://doi.org/10.1007/s11356-023-27331-4
Rosenberg M, Visnapuu M, Saal K, Danilian D, Pärna R, Ivask A, Kisand V (2021) Preparation and characterization of photocatalytically active antibacterial surfaces covered with acrylic matrix embedded nano-ZnO and nano-ZnO/Ag. Nanomaterials 11:3384. https://doi.org/10.3390/nano11123384
Sedefoglu N (2023) Characterization and photocatalytic activity of ZnO nanoparticles by green synthesis method. Optik 288:171217. https://doi.org/10.1016/j.ijleo.2023.171217
Senasu T, Chankhanittha T, Hemavibool K, Nanan S (2021) Visible-light-responsive photocatalyst based on ZnO/CdS nanocomposite for photodegradation of reactive red azo dye and ofloxacin antibiotic. Mater Sci Semicond Process 123:105558. https://doi.org/10.1016/j.mssp.2020.105558
Singh NK, Saha S, Pal A (2013) Solar light-induced photocatalytic degradation of methyl red in an aqueous suspension of commercial ZnO: a green approach. Desalin Water Treat 53(2):1–14. https://doi.org/10.1080/19443994.2013.838520
Srirattanapibul S, Tang IM, Thongmee S (2020) Photo catalytic reduction of Cr6+ by ZnO decorated on reduced graphene oxide (rGO) nanocomposites. Mater Res Bull 122:110705. https://doi.org/10.1016/j.materresbull.2019.110705
Sudapalli AM, Shimpi NG (2023) 3D ZnO sunstar rose nanoflowers morphology with surfactant-assisted reflux exhibit excellent optical properties and photocatalytic activity against hazardous organic dyes. Opt Mater 136:113391. https://doi.org/10.1016/j.optmat.2022.113391
Sun Y, Zhang W, Li Q, Liu H, Wang X (2023) Green and sonogreen synthesis of zinc oxide nanoparticles for the photocatalytic degradation of methylene blue in water. Adv Sensor Energy Mater 2(3):100069. https://doi.org/10.1016/j.asems.2023.100069
Supin KK, Parvathy Namboothiri PM, Vasundhara M (2023) Enhanced photocatalytic actCvity in ZnO nanoparticles developed using novel Lepidagathis ananthapuramensis leaf extract. RSC Adv 13:1497–1515. https://doi.org/10.1039/d2ra06967a
Taha KK, Al Zoman M, Al Outeibi M, Alhussain S, Modwi A, Bagabas AA (2019) Green and sonogreen synthesis of zinc oxide nanoparticles for the photocatalytic degradation of methylene blue in water. Nanotechnol Environ Eng 4:10. https://doi.org/10.1007/s41204-019-0057-3
Tegenaw AB, Yimer AA, Beyene TT (2023) Boosting the photocatalytic activity of ZnO-NPs through the incorporation of C-dot and preparation of nanocomposite materials. Heliyon 9:e20717. https://doi.org/10.1016/j.heliyon.2023.e20717
Wang Q, Zhang J, Song C (2021) Stability and photoelectric nature of polar surfaces of ZnO: Effects of surface reconstruction. Phys Lett A 398:127274. https://doi.org/10.1016/j.physleta.2021.127274
Xu X, Chen Y, Zhang G, Bian H, Zhao M, Ma S (2018) Optical properties and the band-gap variation in diverse Zn1-xSnxO nanostructures. Superlattices Microstruct 123:349–357. https://doi.org/10.1016/j.spmi.2018.09.021
Yan Y, Al-Jassim MM, Wei SH (2023) Oxygen-vacancy mediated adsorption and reactions of molecular oxygen on the ZnO(10¯10) surface. Phys Rev B 72:161307(R). https://doi.org/10.1103/PhysRevB.72.161307
Yang Z, Xie HS, Lin WY, Chen YW, Teng D, Cong XS (2022) Enhanced adsorption−photocatalytic degradation of organic pollutants via a ZIF-67-derived Co−N codoped carbon matrix catalyst. ACS Omega 7:40882–40891. https://doi.org/10.1021/acsomega.2c03846
Yogesh MG, Pankaj SS, Parag RG (2023) Sonocatalytic degradation of chrysoidine R dye using ultrasonically synthesized NiFe2O4 catalyst. Catalysts 13:597. https://doi.org/10.3390/catal13030597
Yu C, Xiong Z, Zhou H, Zhou P, Zhang H, Huang R, Yao G, Lai B (2022) Marriage of membrane filtration and sulfate radical-advanced oxidation processes (SR-AOPs) for water purification: current developments, challenges and prospects. Chem Eng J 433:133802. https://doi.org/10.1016/j.cej.2021.133802
Zhao W, Adeel M, Zhang P, Zhou P, Huang L, Zhao Y, Ahmad MA, Shakoor N, Lou B, Jiang Y, Lynch I, Rui Y (2022) A critical review on surface-modified nano-catalyst application for the photocatalytic degradation of volatile organic compounds. Environ Sci Nano 9:61–80. https://doi.org/10.1039/D1EN00955A
Zhou F, Jingn W, Shi J, Jiang Z, Cheng Y, Gao K (2016) Effects of etching parameters on ZnO nanotubes evolved from hydrothermally synthesized ZnO nanorods. Ceram Int 42:4788–4796. https://doi.org/10.1016/j.ceramint.2015.11.164
Acknowledgements
This research was supported by department of creative innovation in science and technology and Nanomaterials Chemistry Research Unit, Department of Chemistry, Faculty of Science and Technology, Nakhon Si Thammarat Rajabhat University.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
PP and PN collected the dataset and writing—original draft. PR and AK were involved in the conceptualization, data analysis and interpretation, and writing—review and editing. PN and RP assisted in the conceptualization, writing—review and editing, visualization, and supervision. The completed manuscript was reviewed and endorsed by all authors. Corresponding Author: PN. All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Paweena Porrawatkul, Rungnapa Pimsen, Arnannit Kuyyogsuy, Parintip Rattanaburi, and Prawit Nuengmatcha. The first draft of the manuscript was written by Paweena Porrawatkul and Prawit Nuengmatcha and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
We confirmed that this manuscript has not been published elsewhere and is not under consideration by another journal. Ethical approval and informed consent are not applicable for this study.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Editorial responsibility: Nabin Aryal.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Porrawatkul, P., Pimsen, R., Kuyyogsuy, A. et al. Morphology-dependent photocatalytic performance of ZnO nanostructures in organic dye and antibiotic degradation. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05530-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05530-x