Abstract
Growing demand for sustainable and eco-friendly alternatives to petroleum-based polymers has increased the interest in the microalgae-based production of polymers, specifically polyhydroxyalkanoates and polysaccharides. While most studies in microbial polymer production have primarily focused on axenic or genetically engineered cultures of cyanobacteria and eukaryotic algae, little is known about the potential of mixed phototrophic consortia. This study aimed to obtain and evaluate mixed photosynthetic consortia of different origins (natural and residual) as a novel approach for polyhydroxyalkanoates and polysaccharides accumulation. Activated sludge and freshwater samples were collected and inoculated in lab-scale photobioreactors to generate mixed photosynthetic consortia. After a preliminary screening for polymer-accumulating strains under nutrient-unbalanced conditions, the selected strains were subjected to a biphasic strategy (biomass accumulation and nutrient stress) to evaluate their polyhydroxyalkanoates and polysaccharide accumulation. First, cultures were subjected to a nutrient-rich phase to increase the biomass content and then deprived of nutrients (known as the polymer accumulation phase) to evaluate polyhydroxyalkanoates and polysaccharide yield. Findings in this study revealed that the highest polysaccharide yield for activated sludge biomass and freshwater consortia was 460 ± 16 and 320 ± 24 mg glucose g dried biomass−1, respectively. In contrast, the highest polyhydroxyalkanoates accumulation levels for both cultures were calculated at 5 mg polyhydroxyalkanoates g dried biomass−1. The efficacy of nutrient stress as a selective pressure strategy to develop mostly polysaccharides-accumulating consortia was demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Widespread utilization of fossil-based products, including plastics and fossil fuels, is leading to a significant increase in environmental pollution and health-related problems worldwide. In particular, plastics disposal has become a considerable challenge due to the material's non-biodegradable nature and the lack of an adequate recycling infrastructure (OECD 2022), while fossil fuels combustion contributes significantly to greenhouse gas emissions and climate change, exacerbating the already pressing global environmental issues (Keramidas et al. 2021). Thus, an urgent need to explore and implement innovative solutions that can help mitigate the harmful effects of fossil-based products and promote environmentally friendly practices has arisen.
Microalgae-bacteria consortia represent a viable option for generating sustainable and renewable bioproducts (Ray et al. 2022) and wastewater treatment (Aditya et al. 2022). Fundamentally, these microorganisms form symbiotic associations in which (i) microalgae provide bacteria with organic carbon and O2 through photosynthesis, and (ii) bacteria aid in the mineralization of organic matter producing in return CO2 for microalgae photosynthesis. As a result of this exchange and assimilation of dissolved nutrients, biomass rich in various valuable metabolites is produced.
In this context, polyhydroxyalkanoates (PHA) and polysaccharides have recently attracted much attention (Cinar et al. 2020; Rajpoot et al. 2022). These algae-based polymers are accumulated intracellularly and physiologically employed as carbon and energy reserves during metabolic unbalance. PHAs and polysaccharides are attractive materials for bioplastic production as they exhibit mechanical and chemical properties that are relatively similar to traditional plastics (Kalia et al. 2021). They are also adaptable to well-known plastic manufacturing processes, such as injection molding and extrusion, which makes them suitable for large-scale production (Mathiot et al. 2019).
Considering polysaccharides' and PHA's carbon and energy role in microalgae, a stress condition is needed to trigger their accumulation. One of the most common strategies to achieve that is limiting nutrients (Madadi et al. 2021). The limited availability of nitrogen and, to a lesser extent, phosphorous generates an imbalance in the metabolic turnover, which triggers the production of these biopolymers in search of redox balance. It has been demonstrated that the overall lack of nitrogen in the growth medium leads to an increase of both PHA (up to 200 mg g dried biomass−1) and polysaccharides content (up to 480 mg g dried biomass−1) in mixed microalgae consortia (Almeida et al. 2021). More recently, the genus Chlorella was suggested as an interesting strain for PHA and starch photoautotrophic accumulation (Das et al. 2018; Selvaraj et al. 2021).
Nutrient limitation is a species-dependent enhancement strategy that relies on the prevalence of photoautotrophic microorganisms that produce polymers in mixed consortia. The microbial population in these associations is highly dynamic (Quijano et al. 2017; Zhang et al. 2020), which makes them susceptible to contamination with fast-growing cells that do not produce polymers. That poses a challenge when trying to generate high-quality biopolymers from waste effluents, as non-polymer-producing cells can dilute the overall polymer content and reduce the quality of the final product. One approach to address this issue is to sterilize the media and implement highly controlled processes to limit the growth of unproductive microorganisms. However, this strategy can be expensive and may need to be more economically feasible to compete with its petroleum-based counterpart (Arias et al. 2018). Alternatively, selecting a resilient and adaptable inoculum source can increase the feasibility of producing photosynthetic polymers from mixed consortia without compromising their economic and environmental benefits.
Activated sludge (AS) derived from municipal wastewater treatment plants offers a promising source of microorganisms that accumulate polymers (Jayakrishnan et al. 2021; Guleria et al. 2022). A previous research indicates that the intermittent availability of carbon and nitrogen during AS process favors the growth of microorganisms that store polymers. That can result in a net production of 900 mg PHA g dried biomass−1 (Tamis et al. 2014). However, it has also been reported that AS can form predominantly phototrophic consortia taking advantage of dissolved nutrients in the growth medium when exposed to continuous illumination (Quijano et al. 2017; Milferstedt et al. 2017). Considering this information, subjecting an AS inoculum to a two-step enrichment scheme that promotes (i) the formation of phototrophic biomass (nutrient-rich) and (ii) polymer accumulation (nutrient-limitation) would exert an improved selective pressure on the photoautotrophic consortium that stores polymers.
This study aims to evaluate the potential of mixed phototrophic consortia from activated sludge and freshwater, to produce PHA and polysaccharides under a combined nitrogen and phosphorous-limited condition. To the authors' knowledge, this study represents one of the first attempts to evaluate the efficacy of this strategy in sludge-borne eukaryotic algae and cyanobacteria. This study was developed between February and June 2022 at the Laboratory for Research on Advanced Processes for Water Treatment of the Universidad Nacional Autónoma de Mexico, Santiago de Queretaro, Mexico.
Materials and methods
Source of photoautotrophic biomass
Different sources of inoculum were evaluated for obtaining polymer-producing photoautotrophic biomass in samples of different ecological origins. First, samples of wastewater from a treatment plant were collected from Santa Rosa Jauregui in Queretaro, Mexico. The sampling points for primary effluent, and AS were restricted to the upper (clarified) layer of the primary settling tank and a well-mixed segment of the aeration tank. The procedure based on Abouhend et al. (2019) was utilized to generate phototrophic biomass from AS. AS (inoculum) and primary effluent (substrate) were mixed in a 1:10 v/v proportion and transferred into a laboratory-scale glass photobioreactor (PBR). Mean concentrations for selected parameters in wastewater effluent and AS mix are shown in Table 1. The growth of sludge-based eukaryotic algae and cyanobacteria was initiated by continuously illuminating PBR with four 54W fluorescent-light lamps. The illumination provided a photosynthetically active radiation (PAR) of 140 μmol m−2 s−1 on the inner surface of the reactor. Reactors were kept at ambient temperature and stirred at 120 rpm with a StableTemp™ stirring hotplate to ensure complete mixing.
Identification and separation of polymer-storing strains were carried out with a non-axenic Chlorella sorokiniana 211/8 K dominant consortium as a reference, provided by the Department of Civil and Environmental Engineer of the São Paulo State University (UNESP). This photoautotrophic consortium of freshwater origin was initially obtained from the Culture Collection of Algae and Protozoa (CCAP) and cultured in PBR fed with BG-11 growth medium, as it is the most economical and efficient for Chlorella species (Sharma et al. 2016). BG-11 medium composition was the same as in Rueda et al. (2020). The protocol for consortium maintenance is detailed elsewhere (Slompo et al. 2020; Silva et al. 2021).
Obtention of polymer-storing photoautotrophic consortia
Identification of polymer-storing strains
Generated sludge-based and freshwater-based photoautotrophic biomass in the PBR of the previous section were harvested by centrifugation (3000 g, 20 min, 5 °C). After the supernatant was discarded, cell pellets were rinsed with distilled water to remove residual nutrients. Afterwards, these were inoculated in PBR fed with BG-11 limited in nitrogen and phosphorous (BG-11 − NP), as Singhon et al. (2021), described at an initial biomass concentration of 77 ± 7.5 mg VSS L−1 to trigger polysaccharides and PHA accumulation. Cultures were grown and periodically monitored for three weeks maintaining operating conditions (stirring, temperature, and light intensity) as previously described.
The presumptive polymer-accumulating species were identified following a modified Mourão et al. (2020) Nile-red staining procedure. A volume of 1.0 mL of each NP-limited culture suspension was centrifuged weekly. After discarding the supernatant, cell pellets were resuspended in 1.0 mL of Nile-Red dye (100 μg mL−1 dissolved in Dimethyl Sulfoxide (DMSO)) and incubated for 10 min at room temperature. Then, 20 μL of this solution was heat-fixed in glass slides and gently rinsed with distilled water to remove excess dye. PHA, more specifically polyhydroxybutyrates (PHB), granules, and other lipid bodies were visualized at 40X using a fluorescence microscope equipped with an excitation filter (450–490 nm), emission filter (515 nm), and a dichroic beam splitter (500 nm). Morphological identification of polymer accumulating strains was carried out in bright field microscopy and printed (Wehr et al. 2015) and online (Hauer and Komárek 2022) taxonomic databases. Both bright field and fluorescence microscopy were performed using a Nikon ECLIPSE 90i widefield fluorescent microscope.
Separation into polymer-storing consortia
Separation of presumptive polymer accumulating strains was done through serial dilution and micromanipulation. First, samples from each PBR fed with BG-11 − NP were subjected to a fluorescence excitation spectrum of 510–560 nm to facilitate the observation of chlorophyll under a widefield fluorescent microscope (Nikon ECLIPSE 90i). Photoautotrophs were then directly counted using a 0.1 mm improved Neubauer counting chamber and progressively diluted in autoclaved distilled water until a final suspension of 100 cells mL−1 was obtained. Polymer-accumulating algae were then sought based on their identified morphology and aspirated through a micromanipulator constructed by joining the heated tips of glass Pasteur pipettes and pulling them apart while molten. Finally, these were deposited in test tubes containing 1 mL of BG-11 and cultured as previously described. Cultures were successively transferred in PBR with a working volume of 250 mL and 1 L.
Evaluation of growth and enhancement of polymer production in enriched mixed consortia
Experiments were conducted indoors in square methacrylate Flat Panel Photobioreactors (FP-PBR) at room temperature, with a total volume of 15 L and a working volume of 13 L. All FP-PBR were operated in duplicates under batch conditions for 10 d. The reactors were continuously mixed by filtered compressed air injections enriched with 2.0% CO2 at 0.3 VVM for the duration of the experiment. Illumination was continuously supplied using four 54W fluorescent-light, providing a PAR of 140 μmol m−2 s−1. The pH was measured on-line using a Vernier Tris-Compatible Flat pH Sensor and maintained within an 8.0–9.0 range using either 1.0 M NaOH or 1.0 M HCl.
Growth curves, as well as PHA and polysaccharides accumulation kinetics, were evaluated following a two-phase approach. First, inoculums were subjected to a nutrient-rich phase to increase the biomass concentration. FP-PBR fed with BG-11 were inoculated with 70 mg VSS L−1 of homogenized samples of each consortium. The duration of this phase was carried out until a final concentration of approximately 1.0 g VSS L−1 was reached. The second phase enhanced PHA and polysaccharides production through N-limitation, P- limitation, and DIC abundance. Biomass from the nutrient-rich phase was harvested by centrifugation, rinsed with distilled water, and centrifuged once more. After removing residual nutrients from biomass pellets, these were transferred to FP-PBR fed with BG-11 − NP maintaining operating conditions for 29 d as previously described.
Analytical measurements
Nutrients analysis
DIC and DOC concentration was determined using a Shimadzu TOC Model 5050 analyzer, whereas dissolved PO43−–P, NO3−–N, and NO2−–N were measured using a Thermo Scientific DIONEX ICS1500 ion chromatography. NH4+–N was determined using the 10,031 Hach colorimetric method. Samples for analyzing these nutrients were previously filtered through 0.45 μm pore-size filters. Biomass concentration (X) was monitored periodically as Volatile Suspended Solids (VSS) following the standard APHA 2540E (APHA-AWWA-WPCF 2005). The maximum specific growth rate (μ) was calculated via linear regression applied to the logarithmic growth rate obtained from plotting ln X vs. time (days). Biomass productivity (r) was also determined by dividing the difference between X at time t and 0 and subtracting time t minus time 0.
PHA and polysaccharides analysis
PHA and polysaccharides were extracted and measured in duplicates from lyophilized biomass pellets. A volume of 50 mL of mixed liquor samples were collected three days per week, centrifuged, and spread in plastic trays. After freezing for at least 4 h at − 2 ºC, samples were freeze-dried in a Labconco Freezone 6 freeze dryer at − 85 ºC and 0.05 hPa for 24 h.
Extraction and quantification protocol for PHA content was determined utilizing acid methanolysis and gas chromatography (Agilent Technologies 6890B), as reported by Rueda et al. (2022a) with minor modifications. A 2.0 mL volume of acidified (15% v/v H2SO4) methanol and 2.0 mL of chloroform were added to 10 mg of dried biomass in a glass test tube with a Teflon liner screw cap. After vortexing for 1 min, the resulting solution was incubated in a dry bath (Labnet International AccuBlock™) for 2.5 h at 100 °C and cooled at room temperature. Next, 1.0 mL of deionized water was added to each tube and vortexed to facilitate the separation of different solvents by density. The bottom layer (chloroform) was retrieved through a glass Pasteur pipette and filtered through a 0.45 μm pore-size nylon membrane filter. A volume of 1.0 mL from the filtrate was collected, and 1.0 μL was injected into the chromatograph. PHB and PHV (polyhydroxyvalerate) concentrations were determined through a standard curve generated from natural origin Poly[(R)-3-hydroxybutyric acid-co-(R)-3-hydroxyvaleric acid].
Polysaccharides were extracted and measured following a modified phenol–sulfuric method first described by Romero-Frasca et al. (2021). A 1 mL of 1.0 M HCl was added to 1–2 mg of lyophilized biomass in a glass test tube with a Teflon liner screw cap and vortexed for 1 min. After incubating the mixture for 2 h at 100 ºC in a dry bath (AccuBlock™ Labnet International, USA), samples were cooled at room temperature, and polysaccharides were determined as glucose (mg glucose g dried biomass−1) from acid hydrolysis with phenol (5% w/v) and concentrated sulfuric acid.
Results and discussion
Culturing of photosynthetic biomass
The non-axenic Chlorella sorokiniana consortium was grown under photoautotrophic conditions in BG-11 upon arrival to the lab. This initial culture developed a green color after 10 d and was populated by two morphologically different strains. Microscopic observations (Fig. 1a) confirmed that these had a morphological resemblance to Chlorella (dominant) and Scenedesmus genera. Chlorella specimens are commonly described as green-colored, often spherical (3–8 μm in diameter) single cells which lack a flagellum and have a parietal, cup-shaped chloroplast with an easily observable pyrenoid (Ma et al. 2019). On the other hand, Scenedesmus is depicted as elongated, cylindrical (10–20 μm length; 3–6 μm in width) cells with protruding spines or bristles in their poles (Kumar et al. 2021). Strains belonging to this genus are commonly found as single cells or coenobiums consisting of 4 and 8 cells in their exponential and stationary phase, respectively. Overall, recovery of Chlorella -i.e.,, C. sorokiniana- and Scenedesmus strains from freshwater sources has been extensively described (Xin et al. 2010; Ruangsomboon et al. 2013; Kim et al. 2016; Gomaa et al. 2019) and ultimately confirmed in this study.
Microscopic images illustrating the microbial composition of a non-axenic Chlorella consortium observed in bright field visible light microscope at 40X; b predominantly photoautotrophic consortium observed in bright field visible light microscope at 40X; c polymer accumulating Chlorella strains in non-axenic Chlorella consortium observed under fluorescence microscope at 40X after straining with Nile-red dye; d polymer accumulating Chlorella and unicellular cyanobacteria strains in predominantly photoautotrophic consortium observed under fluorescence microscope at 40X after straining with Nile-red dye
A predominantly photoautotrophic consortium from AS was obtained after 14 d of constant illumination. Observations on the microscope revealed that different eukaryotic algae and diatoms strains were obtained with morphological resemblance to Scenedesmus, Nitzschia, Chlorella, Oscillatoria, and Phormidium (Fig. 1b). Morphologically undistinguishable algae, regarded as unicellular cyanobacteria owed to their spherical small cell size (≤ 1 µm diameter) and bright autofluorescence pattern under green light excitation (500–570 nm, image not shown), were also observed. These photosynthetic taxa are commonly prone to survive in polluted aquatic ecosystems due to their intrinsic tolerance to various organic contaminants (Arias et al. 2020). Previous reports have observed that both unicellular (i.e., Synechocystis and Synechoccocus) and motile filamentous (i.e., Oscillatoriales) cyanobacteria, as well as Scenedesmus and Chlorella eukaryotic microalgae, can be successfully obtained from AS and wastewater effluents (Arcila and Buitrón 2016; Milferstedt et al. 2017; Arias et al. 2019; Rueda et al. 2020; Buitrón and Coronado-Apodaca 2022).
Enrichment of consortia with polymer-accumulating algae
An initial qualitative screening (Nile-red) of polymer accumulators confirmed that a selected number of strains were present in both experimental inoculums upon transferal to BG-11 − NP medium. Due to the absence of an organic matter source within the culturing medium, the presence of polymer-accumulating heterotrophic bacteria could be excluded. Chlorella-like single cells (Fig. 1c) and unicellular cyanobacteria (Fig. 1d) emitted a bright red fluorescence within 1–2 weeks of nutrient stress in both photoautotrophic consortia, respectively. Samples of non-axenic Chlorella microalgae and predominantly photoautotrophic consortium samples before transferal to BG-11 − NP were also processed and observed under fluorescence to rule out false positives. Microscopic imaging of these resulted in no observable fluorescence emission (i.e., completely black) for Nile-red dye and a dim, barely visible red emission for non-stressed experimental inoculums (Fig. S1).
Results provided evidence of Nile-red dye's staining efficiency for detecting PHA -specifically polyhydroxybutyrates (PHB)- granules. It can be deduced that Nile-red stained strains can also accumulate polysaccharides, as their catabolism during nutrient stress has been associated with synthesizing PHB granules (Koch et al. 2019). It has been reported that the non-polar oxazone molecules present in Nile-red allow this dye to quickly and strongly bind to the non-polar phospholipidic membrane that covers PHB granules compared to Nile blue and Sudan black (Rumin et al. 2015). The Nile red dye can stain all intracellular lipid-rich bodies in photoautotrophic (Mourão et al. 2020; Meixner et al. 2022) and heterotrophic (Narayanan et al. 2020; Trakunjae et al. 2021) species, including PHB granules. In addition, the total fluorescence emitted during this step could be correlated to the amount of PHB present in the cell culture through analytical methods.
A quantitative analysis of PHA and polysaccharides was performed to confirm the production and accumulation of polymers in these consortia. Separating potentially polymer-accumulating strains was first performed based on the morphology of stained cells via serial dilution and micromanipulation techniques. Cultures were accordingly labeled and referred to from here on as Chlorella sorokiniana dominant consortium (CDC) and mixed photoautotrophic consortium (MPC). Before this test, CDC and MPC were successfully grown in BG-11 for 3–4 weeks and transferred once more to BG-11 − NP medium. The most notable outcome of this experimental test was that polysaccharides accumulation peaked in week 1 with 198 ± 3 mg glucose g dried biomass−1 and 262 ± 4 mg glucose g dried biomass−1 for CDC and MPC, respectively. On the other hand, PHA accumulation peaked after week 2, in which both consortia accumulated 6.7 mg PHA g dried biomass−1 (CDC) and 19 mg PHA g dried biomass−1 (MPC). As a reference, PHA accumulation was also calculated for non-axenic Chlorella consortium and predominantly photoautotrophic consortium before selection in BG-11 − NP, in which no PHA was detected (≤ 0.1 mg PHA g dried biomass−1) after 3 weeks.
Separating unicellular algae and cyanobacteria is a complicated procedure that often requires skilled lab personnel and expensive equipment (Vu et al. 2018). This study showed that high-purity cultures for polymer accumulation could be obtained by combining an enrichment strategy (nitrogen and phosphorous limitation) and serial dilution and micromanipulation techniques. Overall, the N and P limitation allowed the dominance of cells with an intrinsic survival mechanism to store carbon and energy molecules (i.e.,, starch and glycogen), which could be further used to produce PHA (Koch and Forchhammer 2021). As a result, the abundance of non-accumulating strains was gradually reduced. Continuously diluting a sample of these stressed cultures made the physical extraction of dominant, polymer-accumulating strains more efficient. Similar conclusions were drawn by previous studies, which also isolated unicellular and filamentous cyanobacteria (Meixner et al. 2022) for PHA production, as well as Chlorella for biodiesel production (Huang et al. 2019) and microbial ecology investigations (Soares et al. 2022).
Evaluation of growth and polymer production enhancement in enriched mixed consortia
Biomass growth and microbial evolution
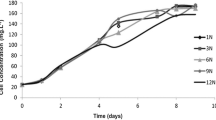
FP-PBR inoculated with CDC and MPC started with a biomass concentration of nearly 70 ± 12 mg VSS L−1 during the biomass accumulation or nutrient-rich phase. Both cultures grew until reaching values of 1.0 ± 0.2 g VSS L−1 by day 12 due to the synergistic actions of photosynthesis and nutrient assimilation. Interestingly, CDC and MPC cultures could grow (Fig. 2) and achieve significant growth kinetics even under nutrient-unbalanced conditions. Biomass concentration, growth rate, and biomass productivity during the PHA accumulation phase in each consortium are shown in Table 2. The observed μ for CDC achieved a similar value to nutrient-limited Chlorella consortia from previous studies, which usually ranged from 0.053 to 0.077 d−1 (Chu et al. 2013). Likewise, μ in MPC was somewhat in range to those observed for wastewater-borne mixed photosynthetic cultures (0.135–0.215 d−1, Arias et al. 2018) used for PHA production under permanent illumination and nutrient stress. Findings in this study, further suggests that nutrient concentration is a critical factor that influences both biomass yield and productivity and, subsequently, polymer yield.
Finally, it should be noted that a yellowish-brown color was observed from day 10 in nutrient-starved FP-PBR inoculated with CDC and MPC (Fig. S2). This quiescence mechanism, called nitrogen chlorosis or bleaching, is a survival strategy commonly triggered during unbalanced nutrient conditions (Koch and Forchhammer 2021; Kumari et al. 2021; Neumann et al. 2021) and a side effect of polymer accumulation. Light harvesting complexes are degraded during nutrient limitation to reduce the photosynthetic activity associated with ATP and NADPH production. Cells favor the synthesis of polysaccharides as a new energy sink for newly fixed CO2 to maintain a redox balance. Eventually, as nutrient limitation becomes persistent over time, cells look for new sources of ATP to maintain a minimum survival level. In some strains, this results in the degradation of polysaccharides and the conversion of the glucose-phosphates intracellular pool into PHA, allowing the cell to stay on standby until nutrients are replenished.
Effect of N-starvation and P-limitation on polymer production
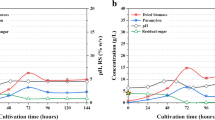
The evolution of polymer accumulation values in nutrient-stressed CDC and MPC cultures are shown in Fig. 3. Polysaccharides content gradually increased in CDC (320 ± 24 mg glucose g dried biomass−1) and MPC (460 ± 16 mg glucose g dried biomass−1) during the first 15 d of nutrient stress. Likewise, PHA content increased and became stable (4.8 mg PHA g dried biomass−1) in MPC at day 13, suggesting that the accumulation of PHA and polysaccharides in Chlorella dominant consortia could not be mutually exclusive. By contrast, CDC showed a somewhat inconsistent PHA accumulation during the operation of nutrient-stressed FP-PBR, reaching up to 3.7 mg PHA g dried biomass −1 after 15 d. To the authors' best knowledge, this is one of the first studies to assess the photoautotrophic PHA production in C. sorokiniana-dominated consortium. Previous reports on this strain have had somewhat contradicting results under mixotrophic conditions, achieving from 4 to 295 mg PHA g dried biomass−1 (Alcántara et al. 2015; Kumari et al. 2022). Results emphasized future studies to develop a photo-mixotrophic mode of operation to assess C. sorokiniana PHA production.
The consensus is that polysaccharides and, eventually, PHA synthesis and accumulation are positively triggered and stabilized over time by removing N sources (Koch and Forchhammer 2021; Sirohi et al. 2021). Nonetheless, findings in our study are a valuable addition to the expanding body supporting the idea that certain P levels lead to a relative reduction in the PHA content, even under N stress. Kamravamanesh et al. (2019) observed that after introducing 9.6 mg P L−1 in polymer accumulating PBR fed with BG-11 N-limited, a 10% decrease in the PHA accumulation yield was observed. Their results suggest that the availability of P prompted the dominant cyanobacterial species (Synechocystis sp.) to prioritize polysaccharides (i.e., glycogen) synthesis over PHA synthesis to sustain cell maintenance. More recent studies further evidenced that even under P concentrations in the range of 1.4–3.0 mg P L−1, PHA production stops altogether and decreases to less than 10 mg PHA g dried biomass−1 (Singhon et al. 2021; Rueda et al. 2022b).
Finally, an analogous study was carried out by Rueda et al. (2020) in mixed consortia using an N and P feast and famine strategy for polymer production. Their research reported an average accumulation of 20 mg PHA g dried biomass−1 and 400 mg glucose g dried biomass−1. They also suggested that different types of C (inorganic for PHA production and organic for PHA/Polysaccharides production) stimulate the accumulation of other polymers under unbalanced nutrient conditions. Although the preparation of BG-11 − NP in the present study does not involve adding organic C sources, and DIC was continuously present in into the growth medium (Fig. 4), an enhanced accumulation of PHA was not observed. Given our improved polysaccharides production, supplementing exogenous organic C by cellular death (due to extended culturing under nutrient stress) and the semi-open operation of FP-PBR are also crucial to control the PHA production process.
Phosphorous and dissolved inorganic carbon (DIC) dynamics for a C. sorokiniana-dominated consortium and b mixed photosynthetic consortium cultures under nutrient-stressed conditions. Bold lines represent time intervals where phosphorous was added. Error bars represent the standard deviation of the mean value
Conclusion
For the first time, a bioprospection of mixed photosynthetic and Chlorella dominant consortia was conducted for photoautotrophic production of polysaccharides and polyhydroxyalkanoates under nutrient stress. Maximum polysaccharides and polyhydroxyalkanoates accumulation values in the Chlorella consortium were 320 ± 24 mg glucose g dried biomass−1 and 4 mg PHA g dried biomass−1 after 15 d of nitrogen starvation, phosphorous limitation, and constant inorganic carbon availability. Conversely, under these conditions, the mixed sludge photosynthetic consortium achieved 460 ± 16 mg glucose g dried biomass−1 and 5 mg PHA g dried biomass−1 after 15 d and 13 d, respectively. Findings in this study, highlight the potential of mixed phototrophic consortia from waste effluents and contaminated sources as a novel and sustainable approach for polyhydroxyalkanoates and polysaccharide accumulation. Although our knowledge of polysaccharide metabolism in algae has expanded considerably in the last years, further studies are needed to fully understand the specific kinetics of starch and glycogen conversion into polyhydroxyalkanoates to sustain a long-term production and ultimately combine waste effluent treatment and bioplastic production.
References
Abouhend AS, Milferstedt K, Hamelin J et al (2019) Growth progression of oxygenic photogranules and its impact on bioactivity for aeration-free wastewater treatment. Environ Sci Technol 54:486–496
Aditya L, Mahlia TMI, Nguyen LN et al (2022) Microalgae-bacteria consortium for wastewater treatment and biomass production. Sci Total Environ 838:155871
Alcántara C, Posadas E, Guieysse B, Muñoz R (2015) Microalgae-based wastewater treatment. In: Kim S-K (ed) Handbook of marine microalgae: biotechnology advances, 1st edn. Academic Press, Amsterdam, pp 439–455
Almeida JR, Serrano E, Fernandez M et al (2021) Polyhydroxyalkanoates production from fermented domestic wastewater using phototrophic mixed cultures. Water Res 197:117101
APHA-AWWA-WPCF (2005) Standard methods for the examination of water and wastewater, 21st ed. American Public Health Association, American Water works Association, Water Pollution Control Federation, Washington D.C
Arcila JS, Buitrón G (2016) Microalgae–bacteria aggregates: effect of the hydraulic retention time on the municipal wastewater treatment, biomass settleability and methane potential. J Chem Technol Biotechnol 91:2862–2870
Arias DM, Uggetti E, García-Galán MJ, García J (2018) Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: effect of nutrients limitation and photoperiods. New Biotechnol 42:1–11
Arias DM, Rueda E, García-Galán MJ et al (2019) Selection of cyanobacteria over green algae in a photo-sequencing batch bioreactor fed with wastewater. Sci Total Environ 653:485–495
Arias DM, García J, Uggetti E (2020) Production of polymers by cyanobacteria grown in wastewater: current status, challenges and future perspectives. New Biotechnol 55:46–57
Buitrón G, Coronado-Apodaca KG (2022) Influence of the solids retention time on the formation of the microalgal-bacterial aggregates produced with municipal wastewater. J Water Proc Eng 46:102617
Chu F-F, Chu P-N, Cai P-J et al (2013) Phosphorus plays an important role in enhancing biodiesel productivity of Chlorella vulgaris under nitrogen deficiency. Bioresour Technol 134:341–346
Cinar SO, Chong ZK, Kucuker MA et al (2020) Bioplastic production from microalgae: a review. Int J Environ Res Public Health 17:1–21
Das SK, Sathish A, Stanley J (2018) Production of biofuel and bioplastic from Chlorella pyrenoidosa. Mater Today Proc 5:16774–16781
Gomaa MA, Refaat MH, Salim TM et al (2019) Identification of green alga Chlorella vulgaris isolated from freshwater and improvement biodiesel productivity via UV irradiation. Microbiol Biotechnol Lett 47:381–389
Guleria S, Singh H, Sharma V et al (2022) Polyhydroxyalkanoates production from domestic waste feedstock: a sustainable approach toward bio-economy. J Clean Prod 340:130661
Hauer T, Komárek J (2022) CyanoDB 2.0 Online database of cyanobacterial genera. Univ. of South Bohemia & Inst. of Botany AS CR. http://www.cyanodb.cz/. Accessed 25 March 2022
Huang ST, Goh JL, Ahmadzadeh H, Murry MA (2019) A rapid sampling technique for isolating highly productive lipid-rich algae strains from environmental samples. Biofuel Res J 6:920–926
Jayakrishnan U, Deka D, Das G (2021) Waste as feedstock for polyhydroxyalkanoate production from activated sludge: implications of aerobic dynamic feeding and acidogenic fermentation. J Environ Chem Eng 9:105550
Kalia VC, Patel SKS, Shanmugam R, Lee JK (2021) Polyhydroxyalkanoates: trends and advances toward biotechnological applications. Bioresour Technol 326:124737
Kamravamanesh D, Slouka C, Limbeck A et al (2019) Increased carbohydrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: bioprocess understanding and evaluation of productivities. Bioresour Technol 273:277–287
Keramidas K, Fosse F, Diaz Vazquez A et al (2021) Global energy and climate outlook 2021: advancing toward climate neutrality. Publications Office of the European Union, Luxembourg
Kim BH, Ramanan R, Kang Z et al (2016) Chlorella sorokiniana HS1, a novel freshwater green algal strain, grows and hyperaccumulates lipid droplets in seawater salinity. Biomass Bioenerg 85:300–305
Koch M, Forchhammer K (2021) Polyhydroxybutyrate: a useful product of chlorotic cyanobacteria. Microb Physiol 31:67–77
Koch M, Doello S, Gutekunst K, Forchhammer K (2019) PHB is produced from glycogen turn-over during nitrogen starvation in Synechocystis sp. PCC 6803. Int J Mol Sci 20:1942
Kumar N, Banerjee C, Jagadevan S (2021) Identification, characterization, and lipid profiling of microalgae Scenedesmus sp. NC1, isolated from coal mine effluent with potential for biofuel production. Biotechnol Rep 30:e00621
Kumari K, Samantaray S, Sahoo D, Tripathy BC (2021) Nitrogen, phosphorus and high CO2 modulate photosynthesis, biomass and lipid production in the green alga Chlorella vulgaris. Photosynth Res 148:17–32
Kumari P, Ravi Kiran B, Venkata Mohan S (2022) Polyhydroxybutyrate production by Chlorella sorokiniana SVMIICT8 under Nutrient-deprived mixotrophy. Bioresour Technol 354:127135
Ma M, Wei C, Wang H et al (2019) Isolation and evaluation of a novel strain of Chlorella sorokiniana that resists grazing by the predator Poterioochromonas malhamensis. Algal Res 38:101429
Madadi R, Maljaee H, Serafim LS, Ventura SPM (2021) Microalgae as contributors to produce biopolymers. Mar Drugs 19:1–27
Mathiot C, Ponge P, Gallard B et al (2019) Microalgae starch-based bioplastics: screening of ten strains and plasticization of unfractionated microalgae by extrusion. Carbohydr Polym 208:142–151
Meixner K, Daffert C, Bauer L et al (2022) PHB Producing cyanobacteria found in the neighborhood—their isolation. Purif Perform Test Bioeng 9:178
Milferstedt K, Kuo-Dahab WC, Butler CS et al (2017) The importance of filamentous cyanobacteria in the development of oxygenic photogranules. Sci Rep 7:17944
Mourão MM, Gradíssimo DG, Santos AV et al (2020) Optimization of polyhydroxybutyrate production by amazonian microalga Stigeoclonium sp. B23. Biomolecules 10:1628
Narayanan M, Kandasamy S, Kumarasamy S et al (2020) Screening of polyhydroxybutyrate producing indigenous bacteria from polluted lake soil. Heliyon 6:e05381
Neumann N, Doello S, Forchhammer K (2021) Recovery of unicellular cyanobacteria from nitrogen chlorosis: a model for resuscitation of dormant bacteria. Microb Physiol 31:78–87
OECD (2022) Global plastics outlook: policy scenarios to 2060. OECD Publishing, Paris
Quijano G, Arcila JS, Buitrón G (2017) Microalgal-bacterial aggregates: applications and perspectives for wastewater treatment. Biotechnol Adv 35:772–781
Rajpoot AS, Choudhary T, Chelladurai H et al (2022) A comprehensive review on bioplastic production from microalgae. Mater Today Proc 56:171–178
Ray A, Nayak M, Ghosh A (2022) A review on co-culturing of microalgae: a greener strategy toward sustainable biofuels production. Sci Total Environ 802:149765
Romero-Frasca E, Velasquez-Orta SB, Escobar-Sánchez V et al (2021) Bioprospecting of wild type ethanologenic yeast for ethanol fuel production from wastewater-grown microalgae. Biotechnol Biofuels 14:93
Ruangsomboon S, Ganmanee M, Choochote S (2013) Effects of different nitrogen, phosphorus, and iron concentrations and salinity on lipid production in newly isolated strain of the tropical green microalga, Scenedesmus dimorphus KMITL. J Appl Phycol 25:867–874
Rueda E, García-Galán MJ, Díez-Montero R et al (2020) Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour Technol 295:122233
Rueda E, Álvarez-González A, Vila J et al (2022a) Inorganic carbon stimulates the metabolic routes related to the polyhdroxybutyrate production in a Synechocystis sp. strain (cyanobacteria) isolated from wastewater. Sci Total Environ 829:154691
Rueda E, Gonzalez-Flo E, Roca L et al (2022b) Accumulation of polyhydroxybutyrate in Synechocystis sp. isolated from wastewaters: effect of salinity, light, and P content in the biomass. J Environ Chem Eng 10:107952
Rumin J, Bonnefond H, Saint-Jean B et al (2015) The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol Biofuels 8:1–16
Selvaraj K, Vishvanathan N, Dhandapani R (2021) Screening, optimization and characterization of polyhydroxybutyrate from fresh water microalgal isolates. Int J Biobased Plast 3:139–162
Sharma AK, Sahoo PK, Singhal S, Patel A (2016) Impact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 6:116
Silva DFS, Speranza LG, Quartaroli L et al (2021) Separation of microalgae cultivated in anaerobically digested black water using Moringa oleifera Lam seeds as coagulant. J Water Process Eng 39:101738
Singhon P, Phoraksa O, Incharoensakdi A, Monshupanee T (2021) Increased bioproduction of glycogen, lipids, and poly(3-hydroxybutyrate) under partial supply of nitrogen and phosphorus by photoautotrophic cyanobacterium Synechocystis sp. PCC 6803. J Appl Phycol 33:1–11
Sirohi R, Lee JS, Yu BS et al (2021) Sustainable production of polyhydroxybutyrate from autotrophs using CO2 as feedstock: challenges and opportunities. Bioresour Technol 341:125751
Slompo NDM, Quartaroli L, Fernandes TV et al (2020) Nutrient and pathogen removal from anaerobically treated black water by microalgae. J Environ Manage 268:110693
Soares F, Trovão J, Portugal A (2022) Phototrophic and fungal communities inhabiting the Roman cryptoporticus of the national museum Machado de Castro (UNESCO site, Coimbra, Portugal). World J Microbiol Biotechnol 38:1–16
Tamis J, Lužkov K, Jiang Y et al (2014) Enrichment of Plasticicumulans acidivorans at pilot-scale for PHA production on industrial wastewater. J Biotechnol 192:161–169
Trakunjae C, Boondaeng A, Apiwatanapiwat W et al (2021) Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1–1 using response surface methodology. Sci Rep 11:1896
Vu CHT, Lee HG, Chang YK, Oh HM (2018) Axenic cultures for microalgal biotechnology: establishment, assessment, maintenance, and applications. Biotechnol Adv 36:380–396
Wehr JD, Sheath RG, Kociolek JP (2015) Freshwater algae of North America: ecology and classification. Academic Press, New York
Xin L, Hong-ying H, Jia Y (2010) Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol 27:59–63
Zhang B, Li W, Guo Y et al (2020) Microalgal-bacterial consortia: from interspecies interactions to biotechnological applications. Renew Sustain Energy Rev 118:109563
Acknowledgements
The technical assistance of Gloria Moreno Rodríguez, Jaime Pérez Trevilla, and Ángel A. Hernández Huerta is greatly appreciated.
Funding
This work was financially supported by CONACYT-ANUIES-ECOS NORD (296514) and CONACYT-OAS-AMEXCID through the grant no. 855766.
Author information
Authors and Affiliations
Contributions
ERF and GB contributed to Conceptualization, Formal analysis and investigation; ERF contributed to Writing—original draft preparation and Methodology; GB contributed to Writing—review and editing, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Editorial responsibility: S. Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Romero-Frasca, E., Buitrón, G. Assessment of polyhydroxyalkanoates and polysaccharides production in native phototrophic consortia under nitrogen and phosphorous-starved conditions. Int. J. Environ. Sci. Technol. 21, 4997–5006 (2024). https://doi.org/10.1007/s13762-023-05332-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05332-7