Abstract
Ecotoxicological tools have proved to be sensitive and appropriate for the evaluation of the effectiveness of treatments used in wastewater treatment plants (WWTP). The objective of this study was to assess the applicability of bioassays and biomarkers to evaluate the efficiency of different treatments throughout WWTP samples[A—raw influent, B—preliminary effluent, C—final effluent, and D—receiving stream], seasonally over 1 year, through a multispecies approach: i) bacterial cell viability [Escherichia coli, Rhodopirellulla rubra, Arthrobacter sp., and Pseudomonas putida]; ii) microalgae Raphidocelis subcapitata and the macrophyte Lemna minor growth inhibition; and iii) microcrustacean Daphnia magna acute and feeding rate assays. Total chlorophyll, malondialdehyde, and proline levels were evaluated in L. minor, and catalase, glutathione-S-transferase activities, and thiobarbituric acid reactive substances levels were quantified in D. magna, after exposure to wastewater samples. Overall, the tested species showed different sensitivities, P. putida = Arthrobacter sp. = R. rubra < R. subcapitata < E. coli = D. magna = L. minor, to the collected samples. The results obtained in D. magna and L. minor assays demonstrated that these organisms can be used in programs for monitoring and environmental assessment of wastewater effluents. The present study demonstrates the usefulness of ecotoxicological tools, with multispecies and different endpoints, to assess the effectiveness of WWTPs. Moreover, it is important to ensure that WWTP implements a monitoring program to minimize the discharge of effluents that compromise the environment in order to guarantee the good ecological quality of the environmental ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growth of the human population, associated with the technological and industrial revolution in the second half of the twentieth century, led to a significant change in the quality of life in society. Today’s generation is driven by the high need for consumption, which reflects the increase in the amount of waste produced and released into the environment (Gargosova and Urminska 2017; Qayoom et al. 2022). These residues include several contaminants of emerging concern (CECs) (Golovko et al. 2021), such as industrial chemicals, pharmaceuticals products, and personal care products, that have been increasingly detected in wastewater systems and natural water bodies (Halling-Sørensen et al. 1998). In this context, wastewater treatment plants (WWTPs) play a fundamental role in the removal of organic matter and reduction in CECs, minimizing their potential adverse effects on aquatic ecosystems. Even though many of these effects are still unknown, it is known that the persistence and the possible bioaccumulation of these compounds in aquatic systems can compromise all the biota that depend on these ecosystems (Halling-Sørensen et al. 1998). Therefore, the main objective of WWTPs is to treat wastewater from domestic and industrial activities, before it is released into the environment (e.g., rivers) (Monte et al. 2016). Wastewaters are complex mixtures of compounds, which include organic and inorganic residues, macronutrients, and chemicals (Gargosova and Urminska 2017).
According to the sensitivity of the receiving environment, treatments along the WWTP can be more or less complex, depending on the characteristics of the influent wastewater and the reuse of the treated effluent (Monte et al. 2016). In a WWTP, the influent can be subjected to four treatment phases: (i) the preliminary treatment (to avoid obstructions of the treatment circuits and contamination) removing coarse solids, sands, oils, and fats; (ii) the primary treatment for removing suspended solid particles; (iii) the secondary treatment, often called biological treatment, consists of reducing organic matter and nutrients in the effluent through the growth of microbiological communities; and (iv) the tertiary treatment is used if the final recipient of the effluents is considered a sensitive area or the objective is to reuse the treated effluents (e.g., for irrigation or washing) (Monte et al. 2016). This latter treatment aims to improve the quality of the final effluent, through the elimination of nutrients (e.g., nitrogen and phosphorus, responsible for eutrophication of waters) and parasites, using disinfection by ultraviolet radiation, ozonation, or treatment with chlorine (Moreira 2014). The evolution of treatments has continued to remove dissolved pollutants present in residual concentrations, such as emerging pollutants. Advanced treatments have been applied to this end, including advanced oxidation processes (AOP), membrane separation processes, and adsorption of activated carbon (Monte and Albuquerque 2010). Despite the increase in knowledge and technology of several treatments, concentrations/levels of compounds (in the order of ng/L and µg/L) of anthropogenic origin are found in wastewater and in the recipient discharge ecosystems (Halling-Sørensen et al. 1998; Kümmerer 2009).
Since the 1970s and 1980s, several countries such as Germany, Belgium, Denmark, Sweden, and Spain have used ecotoxicological tools to monitor the efficiency of the treatments used in WWTP (Power and Boumphrey 2004). This approach allows the assessment of possible impacts of discharges of WWTP effluents on water bodies and ecosystem dynamics, namely the effects on aquatic organisms of different trophic levels (Mendonça et al. 2009, 2013). Flores et al. (2014) and Marinho et al. (2020) used different bacteria (Escherichia coli, Rhodopirellula rubra, Rhodopirellula lusitana, Pseudomonas putida, and Vibrio anguillarum) for the evaluation of toxicity of different metals and pesticides, proving the usefulness of these strains. Vasquez and Fatta-Kassinos (2013) demonstrated that freshwater species such as Daphnia magna and Raphidocelis subcapitata were more sensitive to wastewater effluents than marine species (Artemia salina and Vibrio fischeri). Mendonça et al. (2013) concluded that Vibrio fischeri and Daphnia magna tests are suitable to distinguish and evaluate the effectiveness of treatments used in WWTPs. The same conclusion was drawn by Alkimin et al. (2020), demonstrating the advantages of using L. minor to assess the toxicity of effluents. Thus, ecotoxicological studies have proved to be great tools in assessing the effectiveness of the instruments used in WWTPs for the treatment of wastewater, as well as unmasking the effects (synergism, antagonism, potentiation, or summation) of complex mixtures that may occur (Gargosova and Urminska 2017; Mendonça et al. 2011a).
In addition to similar studies, which focus on one species or several species with the same endpoint to evaluate the toxicity of wastewater effluents, the present study highlights the importance of using a multispecies ecotoxicological approach (bioassays and biomarkers) with several endpoints to assess the toxicity of WWTP effluents. This evaluation was conducted along a WWTP with different treatments: A—raw influent, B—preliminary effluent, C—final effluent, and D—receiving stream. To achieve this goal, organisms of different trophic levels and parameters were assessed: bacteria cell viability assays using Escherichia coli (two different strains), Rhodopirellulla rubra (strain LF2), Arthrobacter sp. (strain FF13), and Pseudomonas putida (strain N3BL); growth inhibition assays using the microalgae Raphidocelis subcapitata and the floating plant Lemna minor; and acute and feeding rate assays using the microcrustacean Daphnia magna. Moreover, subindividual parameters were also evaluated in L. minor [the content of total chlorophyll, malondialdehyde (MDA), and proline] and D. magna [catalase (CAT) and glutathione-S-transferase (GST) activities, and thiobarbituric acid reactive substances (TBARS) levels].
Materials and methods
Characterization of WWTP under study
The Wastewater Treatment Plant under study, located nearby Porto (Portugal), receives domestic and industrial wastewaters, namely from the furniture and textile sectors. For the last years, this WWTP has operated above its capacity (5800 m3/day), leading to the release of effluents with inadequate treatment, or even without treatment discharged to the (final recipient), a small river included in a sensitive area. To increase the efficacy of wastewater treatments and consequently reduce the pollution of the final recipient, the WWTP has undergone expansion and rehabilitation between 2018 and the spring of 2020. This intervention resulted in the installation of a membrane bioreactor (MBR) treatment with an ultrafiltration process (after the conventional activated sludge process) that is expected to increase the total volume of wastewater being treated by 70%.
Sampling procedure
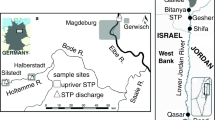
To conduct the present study, four samples were collected along the treatments of the WWTPs, and identified as A, B, C, and D samples, according to the sampling sites (see Fig. S1—supplementary material). Influent samples collected at site A correspond to the reception of the untreated wastewater (raw tributaries). Effluent samples from site B stand for the effluent after going through the preliminary treatment (after the removal of coarse solids, sands, oils, and fats). Sample C stands for the effluent after biological treatment (after undergoing MBR treatment and before the discharge in the river), and site D corresponds to the river sampling site, where the final WWTP effluent is discharged (Fig. S1—supplementary material).
Samples (A, B, C, and D—Fig. S1—supplementary material) were collected seasonally, along the year 2020/2021 (4 sampling periods): autumn (aut20), winter (win20), spring (spr21), and summer (sum21). Several physical and chemical parameters were measured in situ [pH, oxygen (mg/L and %), conductivity (µS/cm), and temperature (°C)], with a multiparametric probe (Multi 3630 IDS SET F). Additionally, 6 L of each sample (A, B, C, and D) were collected with plastic bottles, and transported in the dark at 4 °C to the laboratory, to conduct a chemical characterization of the samples and the laboratory bioassays. Physical and chemical analyses were performed on the sampling day, and bioassays were started within a maximum period of 24 h after the collection of the samples.
Water physical and chemical analysis
In the laboratory, the concentration of nitrites (µg NO2−/L), nitrates (mg NO3−/L), ammonium (mg NH4+/L), and total phosphorus (mg Ptotal/L) were measured in all samples using a Spectroquant Multy Colorimeter. The five-day biochemical oxygen demand (BOD5), as well as the turbidity level, was also quantified according to APHA (1989) guidelines. To determine the dissolved organic carbon (DOC), an aliquot of each sample was filtered through a Whatman GF/C filter (47 mm diameter and 1.2 μm pore) and the absorbance of the filtered sample was measured at λ = 320 nm (Williamson et al. 1999). The total suspended solids (TSS) and the volatile suspended solids (VSS) contents were quantified using three filters obtained from the filtration of each sample (APHA 1989).
Microbiological monitoring
To evaluate the microbiological quality of the samples, several microbiological parameters were calculated. All laboratory procedures for microbiological monitoring were performed under aseptic conditions. For the Escherichia coli counting, the miniaturized method (MUG (4-methylumbelliferyl-beta-D-glucuronide)/EC method) was used (EN ISO 9308-3, 1998). The total microorganisms (aerobic mesophilic microorganisms) was counted, according to the technique of incorporating 1 mL of each sample, and respective dilutions, in Nutrient Agar culture medium (NA), and the incubation occurred at 22 °C and 36 °C, for 68 ± 4 h and 44 ± 4 h, respectively (EN ISO 6222, 1999). The count of the total coliforms and Enterococcus was performed using the membrane filtration technique (Millipore filtration system with 0.45 μm porosity filtering membranes), with 100 mL of each sample was filtered and, if necessary, respective decimal dilutions (EN ISO 7899-2, 2000; EN ISO 9308-1, 2014). The filtering membranes were placed on the surface of the selective culture media (membrane lauryl for total coliforms and Slanetz–Bartley Agar for Enterococcus) and incubated for 24 h at the appropriate temperature.
Test organisms and maintenance conditions
The organisms selected to perform this study belong to several trophic levels that play different key functions in the aquatic food web. A set of bacteria (decomposers)—Arthrobacter sp. (strain FF13), Pseudomonas putida (strain N3BL), Rhodopirellulla rubra (strain LF2), and two different strains of Escherichia coli (ATCC 25922 and an environmental strain), the microalgae Raphidocelis subcapitata, the macrophyte Lemna minor (primary productors), and the Cladocera water flea Daphnia magna (primary consumers) were the species selected to conduct this study. The last three species are considered standard organisms in aquatic ecotoxicology tests (OECD 2004, 2006, 2011), while bacteria represent the basis of the trophic web playing different roles in the environment.
Bacterial strains
Arthrobacter sp. (strain FF13) is a Gram-positive bacterium commonly found in several environments. This aerobic soil bacterium (Hagedorn and Holt 1975) belongs to the phylum Actinobacteria and was isolated from Fucus spiralis collected on a beach in Porto vicinity, Portugal (41°09′ N; 8°40′ W). Escherichia coli is a Gram-negative bacterium that belongs to the phylum Proteobacteria, considered a good indicator of fecal pollution in water environmental samples (Leclerc et al. 2001). In this study, two different strains were used, Escherichia coli ATCC® 25922™ and a strain isolated from an environmental water sample from the Febros river (high ecological relevance since was isolated from natural water) in Avintes, Portugal (Cabral and Marques 2006). Pseudomonas putida (strain N3BL) is a Gram-negative bacterium, easily found in soil and aquatic habitats, that belongs to the phylum Proteobacteria. This bacterium occurs in highly oxygenated environments and was isolated from a marine sponge (Flores et al. 2014). Rhodopirellula rubra (strain LF2) is a marine Gram-negative bacterium, belonging to the phylum Planctomycetes. This bacterium was isolated on the north coast of Portugal, from a biofilm community on the surface of marine macroalgae Laminaria sp. (Lage and Bondoso 2011).
Escherichia coli strains and Pseudomonas putida were cultivated on Nutrient Agar (NA) (3 g/L yeast extract, 5 g/L peptone, 12 g/L agar), while Arthrobacter sp. and Rhodopirellula rubra were cultivated on modified M13 medium (Lage and Bondoso 2011). The growth incubation conditions were performed at 26 °C, except for E. coli strains that were at 37 °C (Flores et al. 2014; Marinho et al. 2020).
Raphidocelis subcapitata
Raphidocelis subcapitata is a freshwater green microalga, used as a bioindicator species in several aquatic ecotoxicological studies, due to its high growth and reproduction rate, and its sensitivity to different toxicants, including effluent samples (OECD 2011; Vasquez and Fatta-Kassinos 2013). This standard microalga was cultured in the Woods Hole MBL medium (Schwartz 1975), under controlled conditions of temperature (23 ± 2 °C) and continuous light. The culture maintenance was performed in the exponential growth phase, being the medium renewed approximately every 7 days (Pinto et al. 2021).
Lemna minor
Lemna minor is a standard floating macrophyte generally used in ecotoxicology (Nunes et al. 2014; OECD 2006). The well-known duckweed is typical of water bodies with a reduced flow or standing water, such as lakes and lagoons (Hillman 1961). The small size, easy handling, high reproductive capacity, and sensitivity to pollutants and wastewater effluents are some of the characteristics that make this species an outstanding test organism (Zaltauskaite et al. 2014). L. minor was cultivated in Steinberg medium and maintained at a temperature of 23 ± 2 °C and continuous light, according to the standard guideline OECD 221 (OECD 2006).
Daphnia magna
Daphnia magna is the standard species widely used to assess the toxicity effects of individual substances or complex mixtures, like domestic and industrial effluents (OECD 2004, 2008; Rodrigues et al. 2021). This Cladocera is easy to maintain in laboratory conditions, reproduces asexually (through parthenogenesis, so with low genetic variability), and plays an important role as a primary consumer in the aquatic food webs (Pinto et al. 2021; Rodrigues et al. 2021). Monoclonal cultures of D. magna were maintained in synthetic water medium "ASTM hard water" (ASTM 1989), supplemented with a standard organic additive, Ascophyllum nodosum extract (Baird et al. 1989) at a temperature of 20 ± 2 °C and photoperiod of 16hL:8hD. The microalgae Raphidocelis subcapitata was used to feed D. magna in the cultures, in a ratio of 3.0 × 105 cells/mL/day.
Bioassays
Acute bacterial cell viability assay
The acute bacterial cell viability assay was performed according to Flores et al. (2014) and Marinho et al. (2020). For the cell viability assays, one milliliter of liquid bacterial culture was centrifuged at 13,400 rpm for 60 s (MiniSpin, Eppendorf). In a flow chamber, the cell pellets were resuspended in 1.5 mL of each sample (A, B, C, and D) and different exposure periods were evaluated (30, 60, 120, 180, 240, 300 min, and 24 h). Cells incubated in Milli Q water were used as controls. After that, 10 µL aliquots were inoculated in the respective growth medium and incubated at an adequate temperature. Growth was checked after 24 h incubation for Arthrobacter sp., E. coli, and P. putida, and after 3 days for R. rubra. Growth and consequently cell viability were assessed according to a growth level scale using a classification ranging from 0 to 4, where 0 stands for the absence of growth (higher effect), and 4 represents the maximum growth (without effect). (For more details, namely figures showing the growth level scale see Flores et al. (2014) and Marinho et al. (2020).)
Raphidocelis subcapitata growth inhibition test
To evaluate the toxicity of the samples (A, B, C, and D) on the microalga R. subcapitata, a growth inhibition test was carried out according to standard guidelines (OECD 2011). A range of dilutions of each sample for each sampling period [20, 40, 60, 80, 100% (direct sample—without dilution)] were prepared with MBL medium. MBL medium was used as a negative control, and a blank (effluent sample) without algae addition was also used in the assay. The results were expressed in yield and used to obtain the effective concentration for 50% organisms [EC50 (72 h)] and corresponding confidence intervals, according to the protocol of OECD (2011). Yield stands for the difference between the biomass (cell densities, cells/mL) at the beginning and the end of the assay for each replicate of controls and treatments (OECD 2011).
Lemna minor growth inhibition test
Lemna minor growth inhibition assays were conducted according to standard guidelines (OECD 2006) with adaptation to a microplate (Marinho et al. 2020). Initially, four dilutions of the samples [A, B, C, and D—20, 40, 60, 80, and 100% (direct sample—without dilution), and for each sampling period] were prepared using the Steinberg medium. Six-well microplates were used to conduct this assay, using four replicates per dilution, each containing 10 mL of the testing dilutions and 4 fronds of L. minor. The control group was conducted with fronds exposed to the Steinberg medium. After the seven days of the exposure period, the final number of fronds was counted. The results were expressed in yield and used to obtain EC50 (7 days) and corresponding confidence intervals (OECD 2006). At the end of the assay, the fronds were washed with distilled water, dried with absorbent paper, and weighed. The fronds are stored in Eppendorf microtubes at – 80 °C for posterior quantification of the total chlorophyll content and determination of specific biochemical endpoints: MDA and proline contents.
The total chlorophyll content was determined according to Pinto et al. (2021). MDA was expressed as µM MDA equivalents/mg fresh weight, which is a measure of the lipid peroxidation in plant cells, and its content was determined by the thiobarbituric acid method as described and adapted by Nunes et al. (2014). The proline content was expressed as mg/mg fresh weight and was determined according to Pinto et al. (2021).
Daphnia magna assays
D. magna acute immobilization assay was performed according to the standard guideline OECD 202 (OECD 2004). A control group (ASTM) and a range of sample dilutions [A, B, C, and D—20, 40, 60, 80, 100% (direct sample—without dilution), and for each sampling period] were prepared. For each condition, four replicates were prepared in glass vessels with 25 mL of sample dilution or ASTM. In each replicate, 5 organisms (24 h old, born between the 3rd and the 5th broods) were added and the assay was performed under controlled conditions of temperature (20 ± 2 °C) and photoperiod (16hL:8hD). The organisms were observed after 48 h of exposure, and dead or immobilized organisms were counted for further determination of EC50 (48 h) values and corresponding confidence intervals.
The feeding rate assay was conducted according to Rodrigues et al. (2021). The assay was conducted in 6-well microplates, and in each replicate, 1.5 × 105 cells/mL of R. subcapitata and 12.5 mL of the sample were added. If no acute toxicity was detected (no mortality), 100% of the samples were tested in feeding rate assay; otherwise, a range of dilutions of that samples were tested to guarantee that the value of the inferior confidence interval of EC50 was not exceeded. In the case of samples C and D, no acute toxicity was recorded in any of the sampling periods (Table 3); therefore, the samples were tested undiluted (100%). Acute toxicity assays evaluate the mortality caused by the samples; on the other hand, the use of feeding rate assays aims to obtain sublethal biological responses, avoiding the mortality of organisms. Five replicates per treatment were used and five neonates (total number of organisms per treatment was 25 neonates), with 4 or 5 days old and born between the 3rd to 5th broods, were added to each replicate. A blank with the sample without organisms was made to account for the potential algal growth during the assay exposure period. After the exposure period, pools of 5 organisms from each treatment were stored in Eppendorf microtubes at -80 °C for posterior biochemical determinations (oxidative stress and lipid peroxidation biomarkers).
Specific oxidative stress and metabolism biomarkers, including catalase (CAT), glutathione-S-transferase (GST) activities, and levels of lipid peroxidation (levels of thiobarbituric acid reactive substances (TBARS)), were determined. Biological samples were sonicated in ice-cold phosphate buffer (50 mM, pH = 7.0 with 0.1% of Triton X-100) and centrifuged at 14,000 rpm for 10 min at 4 °C, according to Rodrigues et al. (2021). Different aliquots were prepared for the different biomarker quantifications.
All biochemical biomarkers were performed in microplates and determined according to Rodrigues et al. (2021), using 3 a 4 replicates depending on the available biomass, at the end of the bioassays. Spectrophotometric readings were performed using a microplate reader (Thermo Scientific, model Multiskan GO, version 1.00.40), with the SkanIt Software 3.2.). All the enzymatic activities were expressed in function of protein content, which was quantified spectrophotometrically (wavelength 595 nm) using γ-globulin as a standard (Bradford 1976).
Catalase (CAT) activity was quantified according to Rodrigues et al. (2021) and expressed as nmoles of H2O2 consumed per minute, per milligram of protein. Glutathione-S-transferase (GST) activity, expressed in millimoles of thioether produced per minute per milligram of protein, was determined by Rodrigues et al. (2021). Lipid peroxidation was measured by the quantification of TBARS, according to Rodrigues et al. (2021), and TBARS levels were expressed as malondialdehyde (MDA) equivalents (mmoles) per milligram of protein.
Statistical analysis
The estimation of EC50 values and respective confidence intervals (CIs) for D. magna were performed by modeling immobilization as binomial data [using the R package “drc”; (Ritz and Streibig 2005)], with a special case of the log-logistic dose–response model, where the asymptotes of the curve are fixed to be 1 (all organisms are immobilized) and 0 (none are immobile), following Ritz (2010).
EC50 values, and respective CIs (using the delta method), were determined by fitting a nonlinear concentration–response toxicity model (LL3) to the R. subcapitata and L. minor yield data using the drc package (Ritz and Streibig 2005) for R software. The yield was modeled as a continuous variable using a three-parameter logistic model, where the lower asymptotes of the curve were fixed to 0, following Ritz (2010).
The data from bioassays were tested for normality by the Shapiro–Wilk test and homogeneity of variances by the Levene test. For all bioassays and biomarkers results, a one-way ANOVA was conducted, followed by the Dunnett test which was carried out to determine differences between the dilutions of samples and the control treatment. The adopted level of significance was 0.05. All the statistical analyses were done using SPSS Statistics v26.
Results and discussion
Physical and chemical parameters
The results from the physical and chemical analysis of the samples are presented in Table 1. According to the decree-law no. 152/97, the discharges from the wastewater treatment plants must satisfy treatment requirements taking into account the sensitivity of the receiving stream. In the case of the studied WWTP, since its effluent is released into a sensitive receiving environment (Ministério do Ambiente 2008) it must be guaranteed that the parameters are within the limit that the legislation considers acceptable (BOD5 < 25 mg/L; TSS < 35 mg/L; Ptotal < 2 mg/L; Table 1) and/or that a minimum percentage reduction concerning the influent (sample A—raw influent) is guaranteed (70–90% BOD5; 90% TSS; 80% Ptotal) (Ministério do Ambiente 1997). Comparing the values obtained with the limit values, we verified that the final effluent (C) only exceeded the value of phosphorus in the sum21 (Ptotal = 2.82 mg/L). However, if we take into account the minimum percentage reduction of the parameters listed above, it is possible to verify that in most samples the treatments were not efficient. As for the minimum percentage of Ptotal reduction, it was never higher than 72.97% [reduction percentage obtained in spr21, between the values obtained in sample A (2.22 mg/L) and sample C (0.60 mg/L)] which shows that the treatments applied were not efficient in removing this nutrient, nor did they comply with the minimum reduction imposed by decree-law no. 152/97 (80%). The same happened with the BOD5 value, which was only efficiently reduced in win20 [reduction percentage 75.8%, between the values obtained in sample A (9.23 mg/L) and sample C (2.23 mg/L)], with an increase being visible in the remaining samples. In this regard, BOD5 is the amount of dissolved oxygen needed (i.e., demanded) by aerobic biological organisms to break down organic material present in a given water sample, during 5 days of incubation at 20 °C, in the darkness. BOD reduction is used as a gauge of the effectiveness of wastewater treatment plants and indicates the short-term impact on the oxygen levels of the receiving water. TSS removal was not effective in win20, exhibiting a reduction only of 30.7% [reduction percentage between the values obtained in sample A (36.1 mg/L) and sample C (25.0 mg/L)].
According to Monte and Albuquerque (2010), the typical composition of untreated urban sewage in Portugal may have a range of variations in TSS (90–430 mg/L), VSS (34–109 mg/L), BOD5 (444–1338 mg/L), ammonium (32–81 mg/L) and Ptotal (3.5–13 mg/L). It is noteworthy that the raw influent (sample A) did not reveal a typical composition of an untreated urban influent, according to the low values for BOD5 (all seasons), TSS and VSS (win20), ammonium (aut20 and win20), and Ptotal (aut20, win20, and spr21).
According to the limits established by the Water Framework Directive (WFD) (European Union 2000), the ecological status of the eco receptor of this study (Ferreira River) was classified as moderate (Agência Portuguesa do Ambiente 2016). Moreover, after the discharge of the final effluent (sample C), in the receiving stream, the ecological status of the river remains moderate (sample D) throughout all sampling periods, due to the non-compliance with parameters such as nutrient content (high values of ammonium and total phosphorus) and BOD5 (Table 1). It is important to emphasize that the final effluent (sample C) contained high amounts of nutrients (NO3−, NH4+, and Ptotal) in most seasons, which can potentiate the degradation of the ecological status of the receiving environment.
After the WWTP rehabilitation, with the implementation of the MBR treatment, it would be expected that the final effluent (sample C) had better quality since several studies already demonstrated that, after MBR treatment, it is possible to obtain an effluent with low values of suspended solids, turbidity, BOD5, and microorganisms (Yang 2013). In the here-presented study, it was observed that the MBR treatments were not able to effectively remove the expected percentages of TSS (> 99%), BOD5 (> 97%), ammonium (80–90%), and total phosphorus (> 62–97%) (Yang 2013). Regarding the TSS, the maximum efficiency reached was 97.8% [obtained in spr21, between the values obtained in sample A (284.7 mg/L) and sample C (5.8 mg/L)]; therefore, the expected removal efficiency was not reached in any of the samples. The same tendency occurs with BOD5, in which is even possible to verify an increase in most sampling (aut20, spr21, and sum21). As for ammonium, the expected removal effectiveness was not achieved only in the Aut20 [effectiveness of 71.8%, between sample A (6.8 mg/L) and sample C (1.92 mg/L)], while the total phosphorus reached the expected effectiveness only in the spr21 [72.97%, between sample A (2.22 mg/L) and sample C (0.60 mg/L)].
Microbiological monitoring
Overall, the number of indicators of fecal contamination (Escherichia coli, fecal enterococci, and total coliforms) was high, except for the number of total coliforms in sample C, in win20 (100/100 mL). According to the literature, on average, a raw influent from a WWTP before any treatment contains from 107 to 109 total coliforms and 106 to 108 fecal coliforms, and depending the treatments, it can decrease by 1 to 3 orders of magnitude (George et al. 2002). In our study, when applied the biological treatment with the membrane bioreactor (MBR), there was a reduction of 2 to 3 orders of magnitude (107 to 104 in aut20, spr21, and sum21; 105 to 103 in win20). However, it would be expected that most of these organisms would be eliminated after all WWTP treatments, as described by Hai et al. (2014). Moreover, coliform bacteria are greatly reduced in the final effluent (sample C), since the removal capacity of the MBR process varies from 105 and 108 coliform bacteria. However, this is not verified in the here-presented study, and a high number of microorganisms was detected in samples C, which reinforces the lack of efficiency of the MBR treatment. The values of the total microbial load for the raw influent (sample A) and the effluent after the preliminary treatment (B), in all seasons, were countless, due to the high load recorded. Regarding the final effluent (C) (except for the win20 sample) and the river sample (D) the microbial load is also countless. The microbial load in the river was higher than sample C but lower than samples A and B. According to the results obtained, the amount of E. coli present in sample D was always higher than the acceptable limit [900 CFU/100 mL, Decree-Law no. 113/2012 (Ministério do Ambiente 2012)]. The same happened with Enterococcus in the aut20 and sum21 samples [330 CFU/100 mL, Decree-Law no. 113/2012 (Ministério do Ambiente 2012)], demonstrating that these samples do not have acceptable bathing quality. These results corroborate the results recorded in the last years where high bacterial concentrations, namely total and fecal coliforms, have been documented (Ministério do Ambiente 2001).
Over the last years, WWTPs with the MBR treatment have revealed some problems with the membranes, such as membrane fouling (Özkan and Uyanık 2017). This phenomenon occurs when the pores of the membranes are totally or partially blocked due to the retention/accumulation of microorganisms, particles, and colloids (Meng et al. 2009). When the ultrafiltration process used at the WWTP is efficient, all particles and microorganisms larger than 0.1–0.01 µm (including bacteria, yeasts, some colloidal particles, and organic macromolecules) should be removed (Jegatheesan and Visvanathan 2014). The obtained results for the microbial load (high microbiological level of the final effluent) can be explained due to changes in the performance of the membranes, fouling or even loss of membrane integrity, compromising the entire process and effectiveness of the treatments, as demonstrated by Hai and Yamamoto (2011).
Bioassays
Acute bacterial viability
Several techniques, traditional (e.g., cultivation in a solid medium) or more advanced (e.g., flow cytometry assay) have been applied to investigate the effects of pollutants on the cellular viability of the microorganisms (e.g., Moghiseh et al. (2019)). In this study, the traditional method of cultivation in a solid medium was used to evaluate the effect of the samples on different bacteria (Flores et al. 2014). No effects were observed after exposure to the samples in the cell viability of Arthrobacter sp., Pseudomonas putida, and Rhodopirellula rubra for all sampling seasons, with maximum growth recorded (level 4). Indeed, several studies already demonstrated that Arthrobacter sp. and Pseudomonas sp. had the ability to degrade a wide variety of compounds (e.g., pharmaceutical products and pesticides) (Marinho et al. 2020), and to reduce the physical and chemical properties of the effluents, such as BOD, DOC, and TSS (Smitha et al. 2012), potentially using the compounds present in the samples as energy sources. Moreover, biological treatment with Arthrobacter sp. and Pseudomonas sp. showed that these bacteria degrade organic and inorganic constituents and reduce ammonium and phosphate levels in wastewater effluents (Smitha et al. 2012). Indeed, Pseudomonas sp. is one of the most common bacteria used in pollutant degradation and bioremediation processes (Sonune and Garode 2015); for example, Pseudomonas putida is commonly used for bioremediation of industrial effluents (Safitri et al. 2015). Unlike the previous species, there are no data in the literature relating Rhodopirellulla rubra to wastewaters. However, it is known that this species belongs to a ubiquitous group and plays an important role in the carbon and nitrogen cycles, being able to use nitrites, nitrates, and ammonium (nutrients that exist in considerable amounts in wastewater) as a source of nitrogen (Flores et al. 2014). It should be noted that Planctomycetes (the phylum of this bacterium) have already been detected in wastewater samples and wastewater treatment bioreactors (Chouari et al. 2003). Fuerst, (2017) described the unique ability, of one group of this phylum, to anaerobically oxidize ammonium (anammox), thus having the possibility to be used as a remediator for nitrogen-rich wastewater.
Table 2 summarizes the effects of the samples on the cell viability of the Escherichia coli strains, an indicator of water quality, commonly used in molecular and ecotoxicological studies (Botelho et al. 2012). The two strains of Escherichia coli showed different sensitivity to the samples under study (A, B, C, and D), revealing higher sensitivity compared to the remaining bacteria tested. In aut20, the growth of E. coli (from the Febros river) was not affected by any sample, while E. coli ATCC 25922 was affected after 30 min of exposure to samples A and B, and after 2 h of sample D. In win20, the growth of the ATCC 25922 strain was inhibited after 240 and 120 min exposure to samples B and D, respectively. However, the growth of E. coli ATCC 25922 was more affected after 30 min of sample A (level growth 0.5), and 100% bacterium death after 1 h of exposure. The same pattern was observed in spr21, for both strains, where an absence of growth was recorded after 5 h (300 min) of exposure. In the case of sample B on spr21, a decrease in cell viability of both strains was observed (level growth 0.5) after 30 min of exposure, and death was observed after 60 and 120 min for E. coli ATCC 25922 and E. coli (from the Febros River), respectively. In the presence of the sample from the river (sample D), the E. coli ATCC 25922 was affected after 30 min and ended up dying after 180 min of exposure, while the other E. coli (from Febros River) showed a gradual decrease in growth until no growth was recorded after 300 min of exposure. Regarding the sum21 sampling, growth inhibition of both E. coli strains was observed after 30 min of exposure to samples A and D (Table 2). Moreover, no growth of E. coli ATCC 25922 and a reduction in the growth of E. coli (from the Febros River) were recorded after 24 h of exposure. Sample B caused a growth decrease of both strains, until growth level 2 (Table 2). A normal growth (level 4) was recorded for the two strains of E. coli for sample C in all seasons, which indicates that the effects on the growth of strains in samples A and B were eliminated by the effectiveness of MBR treatment. Escherichia coli ATCC 25922 is used as a reference strain, known to be sensitive to various compounds, such as antibiotics (ATCC n.d.). On the other hand, E. coli strain, isolated from an environmental and contaminated water sample (Febros River), may have a great ecological relevance since it had been subjected to various anthropogenic pressures. Indeed, Flores et al. (2014) demonstrated that the growth of this strain was not affected by pollutants (arsenic, copper, the fungicide Zidomil and a dish detergent), but was sensitive to phenol and sodium azide. Marinho et al. (2020), found that the growth of this strain was affected only by some compounds, such as Myclobutanil and Azoxystrobin, and only after a longer exposure period (180 and 240 min, respectively). Thus, according to the results obtained in the here-presented study, it was found that after the MBR treatment, samples C were not toxic for E. coli strains in all the sampling periods, revealing some efficiency of the applied treatments. Taking account of the results obtained here, the cell viability assay revealed that, depending on the species used, it can be quick, simple, and applicable in evaluating of toxicity and efficiency of wastewater treatments.
Raphidocelis subcapitata
Table 3 presents the EC50 values and the respective confidence intervals for the acute toxicity of the samples (A, B, C, and D), regarding the different sampling periods (aut20, win20, spr21, and sum21) for Raphidocelis subcapitata. The aut20 sample of the receiving stream (D) and the win20 final effluent (C) were toxic for R. subcapitata. The same happens with samples A, B, and C in spr21, and A and B in sum21 (Table 3). Thus, it is noteworthy that the results do not allow us to establish a toxicity gradient since it was possible to observe different responses, without any clear pattern/trend, for the same sample in different sampling periods.
The growth rate of algae is influenced by physical and chemical factors, such as light, temperature, pH, and nutrients, as well as by the presence of anthropic substances such as heavy metals, pesticides, and personal care products (Larsdotter 2006). In the present study, it was verified that the high amount of ammonium (between 53.6 and 87 mg/L) present in samples A and B may have negatively affected the growth of R. subcapitata, given that the ammonium concentration, according to Larsdotter (2006) should not exceed 20 mg/L. In the case of sample C, the pH of 5.6 may have influenced its growth since the pH in the standard test medium is higher than 7.2 (Schwartz 1975). However, microalgae growth depends on several factors, such as the chemical composition of the sample, and the presence or absence of certain substances (nutrients and organic compounds) that may not only affect their growth but also alter their sensitivity to several toxic substances (Blinova 2004).
Although microalgae are organisms widely used to assess the toxicity of pollutants, some studies have revealed that are not the most suitable model species to be used in ecotoxicological studies with complex mixtures, such as nutrient-rich effluents (Mendonça et al. 2011a). Moreover, colored waters affect algae growth by reducing photosynthetic efficiency due to the degree of water transparency (Gartiser et al. 2010). Berrebaan et al. (2020) found that some municipal effluents were less toxic for Raphidocelis subcapitata (EC50 72 h = 55.4 and 61.83%), due to the complex biological effects–pollutants relationship. Mendonça et al. (2011b) demonstrated that the use of acute assays with different species (Vibrio fischeri, Pseudokirchneriella subcapitata, Thamnocephalus platyurus, Daphnia magna, and Lemna minor) is more sensitive that only test the microalgae, which did not allow the assessment of the toxicity removal efficiency nor to distinguish between the different treatments used. These results are in agreement with the results obtained in this study (Table 2), where it was found that despite being sensitive to some samples in some sampling periods, the microalgae Raphidocelis subcapitata did not prove to be the most suitable organism to evaluate the effectiveness of the treatments used in the WWTP.
Lemna minor
The results obtained in the growth inhibition assay (EC50 values and the respective confidence intervals) with Lemna minor are shown in Table 3. Samples A and B were acutely toxic for this organism in all seasons, while samples C and D only showed toxicity in win20 (EC50 = 93.5% and EC50 = 58.8%, respectively; Table 3). The results of the physical and chemical parameters here measured were not enough to explain the toxic effect recorded on L. minor. Several studies describe wastewater as a complex mixture, which may contain several compounds (impossible to completely quantify) that can inhibit the growth of macrophytes, namely steroids, hormones, heavy metals, and pesticides (Fekete-Kertész et al. 2015). Although L. minor can remove toxic compounds and nutrients from wastewaters, while being often used as a phytoremediator (Alkimin et al. 2020), it has also been demonstrated to be one of the most sensitive organisms used in a battery of ecotoxicological assays involving wastewaters (Blinova 2000, 2004). Indeed, the here-obtained results showed that L. minor was one of the most sensitive species, where acute toxicity was observed in all samples before the biological treatment with MBR (samples A and B), and in win20 also samples C and D showed acute toxicity. Indeed, the macrophyte L. minor has already proved to be a useful organism to assess the toxicity of industrial (Alkimin et al. 2020) and municipal effluents (Zaltauskaite et al. 2011). Zaltauskaite et al. (2014) tested the phytotoxicity of municipal and industrial wastewater effluents in L. minor, and they had obtained slight acute toxicity with an EC50 (7 days) value of 57.13% for untreated wastewater and 47.20% for biologically treated wastewater. Previously, Zaltauskaite et al. (2011) verified that the municipal effluents had no significant adverse effect on the growth of L. minor (recorded as percent inhibition of growth relative to control), after the biological treatment, while the raw influent showed toxicity (EC50 7 days = 55.3%).
Physiological parameters of L. minor have also been used as biomarkers (e.g., total chlorophyll, MDA, and proline content) to assess the toxicity of wastewater (A, B, and C), and river (sample D). Figure S2 (supplementary material) shows the total chlorophyll content after L. minor was exposed to the samples (A, B, C, and D) throughout the different seasons. No pattern was observed in chlorophyll content. In this sense, to highlight the most relevant results, with significantly more consistent differences and not just punctual ones, we decided to remove the significantly less responsive biological parameters, such as total chlorophyll content to the supplementary material section. We consider that these results are important but complementary to those presented in the manuscript, supporting the findings in the parameters that we consider most relevant and reliable (proline and MDA content—Fig. 1). However, in some aut20 dilutions (100% of sample A; 40%, 80%, and 100% of sample B; 60% and 100% of sample C; 20% and 100% of sample D) a decrease in the chlorophyll content was observed (Fig. S2—supplementary material). On the other hand, a significant increase in the chlorophyll content was registered in win20 (92.3% of sample A; 40, 60, and 80% of sample B), spr21 (60% of sample C), and sum21 (40, 60 and 80% of sample C).
Results of lipid peroxidation (MDA content) and proline content in Lemna minor after exposure to samples (%) [A raw influent, B preliminary effluent, C final effluent, and D receiving stream] from different seasons (aut20—autumn of 2020, win20—winter of 2020, spr21—spring of 2021, and sum21—summer of 2021). Data are expressed as mean ± standard error (SE). ANOVA results are also presented at the top of each figure. *Stand for significant differences between treatments and the CTL group (Dunnett test, p < 0.05)
The quantification of chlorophyll content in plants is a parameter rather sensitive to pollution (Fekete-Kertész et al. 2015) and therefore a sensitive indicator of toxicity (Taraldsen and Norberg-King 1990). Despite that, this parameter is not commonly used for assessing the toxicity of wastewater. Alteration in the levels of chlorophylls contents can be used as an indicator of the physiological state of the plant (Nunes et al. 2014) since they may affect the metabolism pathways and physiological functions of the species (Brkanac et al. 2010) which can lead to lower yields of photosynthesis and growth inhibition (Myśliwa-Kurdziel and Strzałka 2002). Moreover, one of the typical symptoms of oxidative stress in plants is a result of the changes in the synthesis/degradation of chlorophylls (Khaleghi et al. 2012). In the here-presented study, the total chlorophyll content of L. minor significantly decreased when exposed to the aut20 samples, even in samples without acute toxicity (see results of C and D, Table 3). These results are in accordance with the results obtained by other authors that reported a reduction in the chlorophyll content when L. minor was exposed to industrial and sewage wastewater (Singh and Singh 2006). In our study, the increase in the total chlorophyll content, when exposed to D samples (in win20, spr21, and sum21), can occur due to a protective mechanism of the plant in response to the stress caused by the effluent. García-Valenzuela et al. (2005) explained that the increase in total chlorophyll occurred due to the development of photosynthetic machinery components (an increase in the number of thylakoids/chloroplasts).
Figure 1 shows the contents of MDA and proline in L. minor after samples exposure. No significant differences were recorded for the concentration of MDA levels in L. minor after exposure to sample A, except for the highest percentages of samples tested at win20 (92.3%). After exposure to sample B, a significant increase in the MDA concentration in L. minor was detected in all the dilutions of aut20, and in the highest effluent percentage tested (99.6%) in win20. A significant increase in the concentration of MDA levels was also recorded in samples C and D of aut20 and sum21, as well as in 100% of sample C of spr21, and 100% of sample D of win20 and spr21. It is noteworthy that although no acute toxicity has been detected in samples C and D in aut20, spr21, and sum21 (Table 3), the quantification of MDA indicates that these water samples affected the metabolic pathways, physiological functions, and potential performance of this macrophyte since membrane damage (lipid peroxidation) was detected. These results show that the quantification of MDA concentration is a sensitive parameter that can be an important indicator of physiological stress (Nunes et al. 2014). Throughout a study with industrial wastewater, the levels of MDA in L. minor increased due to the number of heavy metals found in these effluents (Radić et al. 2011). Zaltauskaite et al. (2011) also described the induction of lipid peroxidation (with an increase in the MDA content) in L. minor when exposed to untreated and biologically treated wastewater.
Regarding the proline content results in Fig. 1, significant punctual changes are observed (with no pattern), except for all dilutions of sample D in spr21, where a significant decrease was observed. A significant increase in proline content was occasionally observed in some samples in the highest dilutions (e.g., A in sum21, C, and D in win20). Proline is an amino acid involved in the regulation of oxidative stress which plays an important role in the redox balance and cell homeostasis (Nunes et al. 2014). The most frequent pattern of response to oxidative stress involves the increase in the proline content (Nunes et al. 2014), the fact that was observed in samples C and D of win20 and sample A of sum21 of the present study. However, the decrease in the proline content in L. minor after exposure of some samples (e.g., 20 and 40% of sample B in spr21, and 100% of sample C in sum21) can be explained by the effects of proline in the direct scavenging of free radicals, and not only in the intermediate process of oxidative stress regulation, as shown by Nunes et al. (2014).
Regarding the here-presented results, L. minor revealed be a good model organism for studies involving wastewater, although some parameters, namely the proline content, proved not to be the best indicator of sensitivity. MDA content proved to be a good sensitive indicator and revealed that the majority of samples C and D (final effluents and samples from the receiving stream) caused oxidative damage in L. minor, even without presenting acute toxicity (Table 3), demonstrating that the WWTP treatments were not entirely efficient. It was also verified that the acute assays with L. minor, complemented with the biochemical assays, allowed the assessment of the efficiency of the treatment, and the toxicity of the samples (Table 3), since the biological responses integrate a complex mixture of compounds and physical and chemical properties beyond those quantified in this work.
Daphnia magna
The results of the D. magna acute immobilization assay are shown in Table 3. No acute toxicity was recorded in win20 for all samples. Raw influent (sample A) showed acute toxicity to D. magna, in aut20 and sum21, while effluent sample B showed toxicity in aut20, spr21, and sum21 seasons. No acute toxicity was recorded in C and D samples in all sampling seasons, which indicates the effectiveness of the treatments of the WWTP in the elimination of the compounds that are toxic to D. magna.
Movahedian et al. (2005) recorded a decrease in D. magna toxicity of the different effluents of a WWTP (from the raw influent to the final effluent), following a reduction of organic and inorganic compounds over the different WWTP treatments. Extensive literature relates the effect of physical and chemical properties of the different effluent samples with D. magna toxicity, stating that large amounts of total suspended solids (TSS > 98 mg/L), high turbidity, and reduced dissolved oxygen (< 3 mg/L) can affect this organism (Chapman et al. 2017; Chen et al. 2012). In the present study, D. magna might have been adversely affected by samples with high TSS (≥ 108 mg/L—sample A and B in aut20), turbidity (≥ 0.757/m—sample A in aut20, and B in aut20 and spr21), and reduced dissolved oxygen concentrations (< 5 mg/L—sample A and B in spr21 and sum21).
Despite being considered more tolerant than smaller cladocerans and other zooplankton species (e.g., Ceriodaphnia sp.) (Blinova 2000), D. magna is considered a model species to monitor the toxicity of different treatments of WWTPs (Mendonça et al. 2013). Besides acute toxicity assessment, a feeding rate assay was carried out to improve the knowledge of the toxicity effects of samples (Fig. S3—supplementary material), since the evaluation of food ingestion (feeding) is one of the most essential biological processes of all organisms. Feeding rate assay has been applied to study the physiological response related to the feeding behavior, of Daphnia magna when exposed to various toxic chemicals and is considered a sensitive tool, in comparison with other standardized tests, for example, toxicity tests involving bioluminescent bacteria and algae growth (Yi et al. 2010). Regarding raw influent (sample A, only in aut20), a significant increase in feeding rate was recorded after exposure to 67% of this sample (Fig. S3—supplementary material). In the win20 and spr21 sampling periods, a significant increase in the feeding rate of D. magna was registered after the exposure to 100 and 74% of sample B, respectively (Fig. S3—supplementary material). Regarding C and D samples, a significant decrease in the feeding rate was verified when Daphnia magna was exposed to 100% in autumn and spring samples (Fig. S3—supplementary material). This can be explained by the fact that cladocerans, such as Daphnia magna, are non-selective filter-feeder organisms, and use colloidal particles, bacteria (Gellis and Clarke 1935), algae, flagellates, and detritus as food sources (Marinho et al. 2018).
Serra et al. (2019) considered that organisms such as D. magna can be used as an alternative method to tertiary treatments in WWTPs, as they are capable of removing suspended particles (Shiny et al. 2005) and emerging pollutants (Matamoros et al. 2012). These authors also concluded that during a short exposure period (24 h), these organisms may be able to remove small particles, reduce nutrients, and improve effluent quality. Nevertheless, with the increase in the exposure time (acute exposure of 48 h) and the dilution of effluents, we verified that these could become toxic, causing death or immobilization (Table 3). On the other hand, in aut20, samples C and D do not cause lethal acute toxicity, and the feeding rate decreased demonstrating that the sublethal feeding response is more sensitive than lethal acute toxicity. These results are in agreement with the results obtained by Yi et al. (2010) that verified that textile and dye effluents inhibited D. magna feeding rate, despite not revealing toxicity after 48 h of exposure (no acutely toxic). Currently, the combined study of toxicological tests of post-exposure feeding of D. magna with biochemical determinations has increased, since they allow the evaluation of the effects of complex mixtures and may complement studies on ecological monitoring of water quality (Rodrigues et al. 2021). To highlight the most relevant results and figures with more detailed information, with significantly more consistent differences, feeding rate assay results were included in the supplementary material section (Fig. S3). We consider that these results are important but complementary to those presented in the manuscript.
To perceive the effects that occurred at the cellular level, different specific oxidative stress and metabolism biomarkers were determined in D. magna after exposure (24 h) to WWTP samples (Figs. 2, S4). When the organisms are exposed to compounds that increase the formation of reactive oxygen species (ROS), the activity of this antioxidant defense enzyme can be altered resulting in oxidative stress (Rodrigues et al. 2021). D. magna exposed to raw influent (sample A) showed a significant decrease in the CAT activity (Fig. 2) in the presence of the highest percentages tested in aut20 and the presence of 36 and 78% of the sum21 sample. A significant increase in CAT activity was observed only at 100% of the spr21 sample and the lowest percentage tested in the sum21 (20%) in sample A. Regarding sample B, a significant decrease in the CAT activity was recorded in spr21 and most aut20 samples, except for the highest percentage of aut20 (96%) and all percentages tested in sum21. D. magna exposure to 100% of the sample C showed a significant increase in CAT activity in all sampling periods, except for the winter season. Organisms exposed to samples from the river (sample D) showed an increase in CAT activity in all sampling periods, except for aut20, where a significant decrease was recorded.
Biochemical results of CAT and GST activities of Daphnia magna, after exposure to the different samples (%) [A raw influent, B preliminary effluent, C final effluent, and D receiving stream] from different seasons (aut20—autumn of 2020, win20—winter of 2020, spr21—spring of 2021, and sum21—summer of 2021). Data are expressed as mean ± standard error (SE). ANOVA results are also presented at the top of each figure. *Stand for significant differences between treatments and the CTL group (Dunnett test, p < 0.05)
Glutathione-S-transferase (GST) are a group of isoenzymes capable of turning toxic compounds more easily excretable by catalyzing their conjugation with reduced glutathione (GSH), playing an important role in detoxification and antioxidant defense (Rodrigues et al. 2021). The activity of GSTs (Fig. 2) significantly increased in sample A of spr21 and sum21, as well as in the lowest percentages tested in aut20. As for sample B, a significant decrease in GST activity was detected in the lowest percentage (21%) of aut20, while the opposite occurred in the highest percentages tested (66 and 96%) of aut20 and the lowest (21%) of sum21. No significant effects in GST activity were obtained when D. magna was exposed to the final effluent (sample C), except for the aut20 sample where a significant increase was observed at 100% of this effluent. The river samples (D) caused an increase in GST activity in all sampling periods except for spr21 where no significant differences were detected.
Figure S4 (supplementary material) shows the results of lipid peroxidation, in Daphnia magna after 24 h exposure to the samples (A, B, C, and D) for all sampling periods. Higher levels of TBARS indicate a higher cell/tissue toxicity, through the induction of lipid peroxidation (Rodrigues et al. 2021). According to the here-obtained results, a decrease in TBARS levels in the presence of sample A of aut20 and win20 was recorded, while in the spr21 and lowest dilutions (20 and 40%) of sum21 samples a significant increase in TBARS was observed (Fig. S4—supplementary material). D. magna exposure to sample B showed a decrease in TBARS levels in all seasons, apart from the highest percentage (96%) of aut20, as well as win20 and sum21 samples. Regarding sample C, a significant increase in TBARS levels was observed at 100% of samples in aut20 and sum21, while a significant decrease was observed in win20. A significant increase in the TBARS levels of D. magna after being exposed to effluent sample D was observed in all seasons (Fig. S4—supplementary material).
Kim et al. (2012) determined the activity of antioxidant enzymes such as CAT, SOD (superoxide dismutase), and GPx (glutathione peroxidase) to assess the oxidative stress caused by industrial effluents in Daphnia magna and Moina macroscopa, after a 24 h exposure. These authors also verified that despite the increased activity of these enzymes, oxidative stress was detected even in samples where acute toxicity was not observed, as it happened in the present study with samples C (aut20 and sum21) and D (all seasons) (Fig. 2).
According to the here-obtained results, the activity of CAT and GSTs (Fig. 2) was crucial to preventing oxidative damage in the cell membranes of organisms exposed to sample A (aut20 and win20), B (aut20 and spr21) and C (win20 and spr21). Regarding that in the main article, we include only the results of CAT and GST activities (Fig. 2) that show to be the most sensitive biochemical parameters in the evaluation of the toxicity and efficiency of wastewater treatments. The results of a potential consequence of oxidative stress, TBARS levels results were included in the supplementary material (Fig. S4), since we can infer that the alterations in TBARS levels, in part, may be associated with functional inefficiency (an increase in TBARS levels) or efficacy (a decrease in TBARS levels) of antioxidant defenses (e.g., CAT and GSTs). In the remaining samples, where oxidative damage was verified, despite the mobilization of detoxification and antioxidant defense pathways, these mechanisms were not enough to neutralize and prevent lipid peroxidation (Figs. 2, and S4). Although there are no studies about the evaluation of the biochemical effects of the wastewater in D. magna, other studies reported oxidative stress and metabolic disturbances in other aquatic organisms that despite being phylogenetically different species, they have similar biochemical mechanisms and metabolic pathways. Cazenave et al. (2014) demonstrated that exposure, for 96 h, to untreated domestic wastewater induced oxidative stress (e.g., increase the activity of GSTs) in vital organs (brain, liver, and kidney), but not in the gills of the fish Prochilodus lineatus (freshwater fish). On the other hand, the increase in glutathione reductase and catalase activities prevented lipid peroxidation and consequently the oxidative stress in the gills of P. lineatus. High levels of antioxidant activities enzymes (glutathione reductase, catalase) were also reported in rainbow trout (Oncorhynchus mykiss) after exposure to municipal wastewater, for 5 days (Sturve et al. 2008) and in Lasmigona costata (freshwater mussel) when exposed to municipal effluents rich in pharmaceutical and personal care products (PPCPs) (Gillis et al. 2014).
Conclusion
The use of the ecotoxicological approach can be an important approach for a more complete evaluation of wastewater effluents. Despite physical and chemical characterization showing that the WWTP often meets the requirement for discharge, the microbiological characterization demonstrated that the treatments were not efficient, exhibiting a high number of fecal contamination indicators, such as Escherichia coli, fecal enterococci, and total coliforms in the final effluent. Although the application of the new treatment (MBR) partially reduced the microbial load, it was not able to eliminate what would be expected for this type of treatment.
The here-obtained results showed that the toxicity of effluent samples depends on the treatments at the WWTP and the sensitivity of the species tested. The ecotoxicological assays with the two strains of Escherichia coli, Lemna minor and Daphnia magna showed sensitivity to the different samples of WWTP (e.g., samples A and B). Overall, the final effluent from the WWTP and the sample of the river (samples C and D, respectively) do not show acute toxicity to D. magna and L. minor. However, biochemical disturbances were detected in the presence of these effluents, demonstrating that the toxicity was not effectively eliminated with the WWTP treatments used. The sensitivity of D. magna and L. minor to these samples (C and D) allowed the evaluation of an integrated biological response to a complex mixture, demonstrating how they can be used as an early warning of toxicity. These results revealed the importance in incorporated this approach (different endpoints and species) into programs for monitoring and environmental assessment of wastewater effluents.
The results of the present study were expected and a little uncertain, since this WWTP is still undergoing adjustments after the rehabilitation works. It is also noteworthy that at this stage, almost 1 year after, the WWTP should already be treating effluents more efficiently. These environmental problems can be transversal to many other WWTPs, and the present study demonstrated that an ecotoxicological approach is a great tool to assess the impact of wastewaters effluents, contributing to a more precise establishment of discharge conditions, and maximizing environmental protection, aiming at the good ecological quality of the receiving ecosystems. The results of our work encourage further studies on the applicability of ecotoxicological tools, including more sampling campaigns, and the use of a higher number of bioindicators needed, for the ecological evaluation of WWTP efficiency (e.g., to establish thresholds), and its integration in programs of management.
Availability of data and materials
All the data produced in this study are presented in the manuscript.
References
Agência Portuguesa do Ambiente (2016) Plano de gestão de região hidrográfica, parte 5—objetivos. Anexo II.3, Região hidrográfica do Douro (RH3). https://apambiente.pt/sites/default/files/_SNIAMB_Agua/DRH/PlaneamentoOrdenamento/PGRH/2016-2021/PTRH3/PGRH_2_RH3_Parte5_AnexoII_3.pdf
Alkimin GD, Paisio C, Agostini E, Nunes B (2020) Phytoremediation processes of domestic and textile effluents: Evaluation of the efficacy and toxicological effects in Lemna minor and Daphnia magna. Environ Sci Pollut Res 27(4):4423–4441. https://doi.org/10.1007/s11356-019-07098-3
APHA (1989) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association, Water Environment Federation. American Public Health Association, Washington
ASTM (1989) Standard practice for conducting acute toxicity tests with fishes, macroinvertebrates, and amphibians. In: Reports E 729-80, vol 11.04
ATCC (2021) Escherichia coli (Migula) Castellani and Chalmers|ATCC. https://www.atcc.org/products/25922. Accessed 30 Jun 2021
Baird DJ, Soares A, Girling A, Barber I, Bradley M, Calow P (1989) The long-term maintenance of Daphnia magna straus for use in ecotoxicology test: problems and prospects. In: 1st European conference on ecotoxicology, pp 144–148
Berrebaan I, Montassir L, Alami M, Saadallah M, Bessi H (2020) The integration of bioanalytical approaches in the assessment of municipal wastewater treatment plant performances. J Water Environ Technol 18(6):383–397. https://doi.org/10.2965/jwet.18-092
Blinova I (2000) The perspective of microbiotests application to surface water monitoring and effluent control in Estonia. Environ Toxicol: Int J 15(5):385–389. https://doi.org/10.1002/1522-7278
Blinova I (2004) Use of freshwater algae and duckweeds for phytotoxicity testing. Environ Toxicol: Int J 19(4):425–428. https://doi.org/10.1002/TOX.20042
Botelho R, Froes C, Santos J (2012) Toxicity of herbicides on Escherichia coli growth. Braz J Biol 72(1):141–146. https://doi.org/10.1590/S1519-69842012000100016
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brkanac SR, Stipaničev D, Širac S, Glavaš K, Pevalek-Kozlina B (2010) Biomonitoring of surface waters using duckweed (Lemna minor L.). Fourth International Scientific Conference BALWOIS 2010, Republic of Macedonia
Cabral JP, Marques C (2006) Faecal coliform bacteria in febros river (Northwest Portugal): temporal variation, correlation with water parameters, and species identification. Environ Monit Assess 118(1–3):21–36. https://doi.org/10.1007/s10661-006-0771-8
Cazenave J, Bacchetta C, Rossi A, Ale A (2014) Deleterious effects of wastewater on the health status of fish: a field caging study. Ecol Ind 38:104–112. https://doi.org/10.1016/j.ecolind.2013.10.029
Chapman PM, Hayward A, Faithful J (2017) Total suspended solids effects on freshwater lake biota other than fish. Bull Environ Contam Toxicol 99(4):423–427. https://doi.org/10.1007/S00128-017-2154-Y
Chen L, Zhang G, Zeng Y (2012) Influences of temperature, pH and turbidity on the behavioral responses of Daphnia magna and Japanese medaka (Oryzias latipes) in the biomonitor. Proced Environ Sci. https://doi.org/10.1016/j.proenv.2012.01.007
Chouari R, le Paslier D, Daegelen P (2003) Molecular evidence for novel planctomycete diversity in a municipal wastewater treatment plant. Appl Environ Microbiol 69(12):7354–7363. https://doi.org/10.1128/AEM.69.12.7354-7363.2003
ISO 9308-3 (1998) Water quality—Detection and enumeration of Escherichia coli and coliform bacteria—Part 3: miniaturized method (most probable number) for the detection and enumeration of E. coli in surface and wastewater
ISO 6222 (1999) Water quality—Enumeration of culturable micro-organism—Colony count by inoculation in a nutrient agar culture medium
ISO 7899-2 (2000) Water quality—Detection and enumeration of intestinal enterococci—Part 2: Membrane filtration method
ISO 9308-1 (2014) Water quality—Enumeration of Escherichia coli and coliform bacteria—Part 1: membrane filtration method for waters with low bacterial background flora
European Union (2000) Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for Community action in the field of water policy. OJ L327, 22.12.2000.
Fekete-Kertész I, Kunglné-Nagy Z, Gruiz K (2015) Assessing toxicity of organic aquatic micropollutants based on the total chlorophyll content of Lemna minor as a sensitive endpoint. Period Polytech Chem Eng 59(4):262–271. https://152.66.114.10/ch/article/view/8077
Flores C, Catita JAM, Lage OM (2014) Assessment of planctomycetes cell viability after pollutants exposure. Antonie Van Leeuwenhoek, Int J Gener Mol Microbiol 106(2):399–411. https://doi.org/10.1007/s10482-014-0206-4
Fuerst JA (2017) Planctomycetes—new models for microbial cells and activities. Microb Resour: Funct Exist Nat Appl 1–27:1–27. https://doi.org/10.1016/B978-0-12-804765-1.00001-1
García-Valenzuela X, García-Moya E, Rascón-Cruz Q, Herrera-Estrella L, Aguado-Santacruz GA (2005) Chlorophyll accumulation is enhanced by osmotic stress in graminaceous chlorophyllic cells. J Plant Physiol 162(6):650–661. https://doi.org/10.1016/j.jplph.2004.09.015
Gargosova H, Urminska B (2017) Assessment of the efficiency of wastewater treatment plant using ecotoxicity tests. Fresen Environ Bull 26(1):56–62
Gartiser S, Hafner C, Hercher C, Kronenberger-Schäfer K, Paschke A (2010) Whole effluent assessment of industrial wastewater for determination of bat compliance. Environ Sci Pollut Res 17(4):856–865. https://doi.org/10.1007/S11356-009-0289-Z
Gellis SS, Clarke GL (1935) Organic matter in dissolved and in colloidal form as food for Daphnia magna. Physiol Zool 8(2):127–137. https://doi.org/10.1086/PHYSZOOL.8.2.30152386
George I, Crop P, Servais P (2002) Fecal coliform removal in wastewater treatment plants studied by plate counts and enzymatic methods. Water Res 30(10):2607–2617. https://doi.org/10.1016/S0043-1354(01)00475-4
Gillis PL, Higgins SK, Jorge MB (2014) Evidence of oxidative stress in wild freshwater mussels (Lasmigona costata) exposed to urban-derived contaminants. Ecotoxicol Environ Saf 102(1):62–69. https://doi.org/10.1016/J.ECOENV.2013.12.026
Golovko O, Örn S, Sörengård M, Frieberg K, Nassazzi W, Lai FY, Ahrens L (2021) Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci Total Environ 754:142122. https://doi.org/10.1016/J.SCITOTENV.2020.142122
Hagedorn C, Holt JG (1975) A nutritional and taxonomic survey of Arthrobacter soil isolates. Can J Microbiol 21(3):353–361. https://doi.org/10.1139/m75-050
Hai F, Riley T, Shawkat S, Magram S, Yamamoto K (2014) Removal of pathogens by membrane bioreactors: a review of the mechanisms, influencing factors and reduction in chemical disinfectant dosing. Water 6(12):3606–3630. https://doi.org/10.3390/w6123603
Hai F, Yamamoto K (2011) Membrane biological reactors: theory, modeling, desing, management and applications to wastewater reuse. In: Hai F, Yamamoto K, Lee C (eds). IWA. http://ro.uow.edu.au/cgi/viewcontent.cgi?article=2198&context=scipapers
Halling-Sørensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment—a review. Chemosphere 36(2):357–393. https://doi.org/10.1016/S0045-6535(97)00354-8
Hillman WS (1961) The Lemnaceae, or duckweeds. Bot Rev 27(2):221–287. https://doi.org/10.1007/bf02860083
Jegatheesan V, Visvanathan C (2014) Process fundamentals: from conventional biological wastewater treatment to MBR. In: Hai F, Yamamoto K, Lee CH (eds) Membrane biological reactors: theory, modeling, design, management and applications to wastewater reuse. IWA, pp 29–54. https://iwaponline.com/ebooks/chapter-pdf/582308/9781780409177_0035.pdf
Khaleghi E, Arzani K, Moallemi M (2012) Evaluation of chlorophyll content and chlorophyll fluorescence parameters and relationships between chlorophyll a, b and chlorophyll content index under water stress in Olea europaea cv. Dezful. World Acad Sci, Eng Technol 6:1154–1157
Kim SB, Kim WK, Chounlamany V, Seo J, Yoo J, Jo H, Jung J (2012) Identification of multi-level toxicity of liquid crystal display wastewater toward Daphnia magna and Moina macrocopa. J Hazard Mater 227:327–333. https://doi.org/10.1016/j.jhazmat.2012.05.059
Kümmerer K (2009) The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. In: Journal of environmental management, vol 90, issue 8, pp 2354–2366. https://doi.org/10.1016/j.jenvman.2009.01.023
Lage OM, Bondoso J (2011) Planctomycetes diversity associated with macroalgae. FEMS Microbiol Ecol 78(2):366–375. https://doi.org/10.1111/j.1574-6941.2011.01168.x
Larsdotter K (2006) Wastewater treatment with microalgae—a literature review. Vatten 62(1):31. https://www.tidskriftenvatten.se/wp-content/uploads/2017/04/48_article_2125.pdf
Leclerc H, Mossel DAA, Edberg SC, Struijk CB (2001) Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. In: Annual review of microbiology, vol 55, issue 1, pp 201–234. https://doi.org/10.1146/annurev.micro.55.1.201
Marinho MC, Diogo BS, Lage OM, Antunes SC (2020) Ecotoxicological evaluation of fungicides used in viticulture in non-target organisms. Environ Sci Pollut Res 27(35):43958–43969. https://doi.org/10.1007/S11356-020-10245-W/FIGURES/2
Marinho MC, Lage OM, Catita J, Antunes SC (2018) Adequacy of planctomycetes as supplementary food source for Daphnia magna. Antonie Van Leeuwenhoek 111(6):825–840. https://doi.org/10.1007/s10482-017-0997-1
Matamoros V, Sala L, Salvadó V (2012) Evaluation of a biologically—based filtration water reclamation plant for removing emerging contaminants: a pilot plant study. Biores Technol 104:243–249. https://doi.org/10.1016/j.biortech.2011.11.036
Mendonça E, Picado A, Cunha MA, Catarino J (2011a) Environmental management of wastewater treatment plants—the added value of the ecotoxicological approach. In: Broniewicz E (ed) Environmental management in practice. InTech, pp 411–424. https://doi.org/10.5772/18389
Mendonça E, Picado A, Paixão S, Barbosa M, Cunha MA, Silva L (2011b) The role of ecotoxicological evaluation in changing the environmental paradigm of wastewater treatment management. In: 6th Dubrovnik conference on sustainable development of energy, water and environment systems
Mendonça E, Picado A, Paixão SM, Silva L, Barbosa M, Cunha MA (2013) Ecotoxicological evaluation of wastewater in a municipal WWTP in Lisbon area (Portugal). Desalin Water Treat 51(19–21):4162–4170. https://doi.org/10.1080/19443994.2013.768021
Mendonça E, Picado A, Paixão SM, Silva L, Cunha MA, Leitão S, Moura I, Cortez C, Brito F (2009) Ecotoxicity tests in the environmental analysis of wastewater treatment plants: case study in Portugal. J Hazard Mater 163(2–3):665–670. https://doi.org/10.1016/j.jhazmat.2008.07.012
Meng F, Chae SR, Drews A, Kraume M, Shin HS, Yang F (2009) Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res 43(6):1489–1512. https://doi.org/10.1016/J.WATRES.2008.12.044
Ministério do Ambiente (1997) Decreto-Lei no 152/97 de 19 de Junho. In: Diário da República: vols I-Série A
Ministério do Ambiente (2001) Decreto regulamentar no 19/2001 de 10 de agosto. In: Diário da República: Vols I-Série B
Ministério do Ambiente (2008) Decreto-Lei no 198/2008 de 8 de outubro. In: Diário da República: Vols I-Série A. https://www.inag.pt
Ministério do Ambiente. (2012). Decreto-Lei no 113/2012 de 23 de maio. In Diário da República.
Moghiseh Z, Rezaee A, Ghanati F (2019) Metabolic activity and pathway study of aspirin biodegradation using a microbial electrochemical system supplied by an alternating current. Chemosphere 232:35–44. https://doi.org/10.1016/j.chemosphere.2019.05.186
Monte H, Albuquerque A (2010) Reutilização de águas residuais. In: ERSAR (ed). https://ubibliorum.ubi.pt/handle/10400.6/1144
Monte H, Santos M, Barreiros A, Albuquerque A (2016) Tratamento de águas residuais – Operações e processos de tratamento físico e químico. CT05, ERSAR, Lisboa, Portugal, 544 p. (ISBN 978 989 8360 32 8).
Moreira C (2014) Etar. Rev Ciênc Elem 2(2):1–3. https://doi.org/10.24927/rce2014.146
Movahedian H, Bina B, Asghari GH (2005) Toxicity evaluation of wastewater treatment plant effluents using Daphnia magna. J Environ Health Sci Eng 2(2):1–4. http://ijehse.tums.ac.ir/index.php/jehse/article/view/33
Myśliwa-Kurdziel B, Strzałka K (2002) Influence of metals on biosynthesis of photosynthetic pigments. In: Physiology and biochemistry of metal toxicity and tolerance in plants. Springer, Dordrecht, pp 201–207. https://doi.org/10.1007/978-94-017-2660-3_8
Nunes B, Pinto G, Martins L, Gonçalves F, Antunes SC (2014) Biochemical and standard toxic effects of acetaminophen on the macrophyte species Lemna minor and Lemna gibba. Environ Sci Pollut Res 21(18):10815–10822. https://doi.org/10.1007/s11356-014-3059-5
OECD (2004) Test no. 202: Daphnia sp. acute immobilisation test. https://doi.org/10.1787/9789264069947-en
OECD (2006) Test no. 221: Lemna sp. growth inhibition test. https://doi.org/10.1787/9789264016194-en
OECD (2008) Test no. 211: Daphnia magna reproduction test. OECD. https://doi.org/10.1787/9789264070127-en
OECD (2011) Test no. 201: Freshwater Alga and Cyanobacteria, growth inhibition test. https://doi.org/10.1787/9789264069923-en
Özkan O, Uyanık İ (2017) Effect of membrane type for the treatment of organized industrial zone (OIZ) wastewater with a membrane bioreactor (MBR): batch experiments. Water (switzerland) 9(8):592. https://doi.org/10.3390/W9080582
Pinto I, Rodrigues S, Lage OM, Antunes SC (2021) Assessment of water quality in Aguieira reservoir: ecotoxicological tools in addition to the Water Framework Directive. Ecotoxicol Environ Saf 208:111583. https://doi.org/10.1016/j.ecoenv.2020.111583
Power EA, Boumphrey RS (2004) International trends in bioassay use for effluent management. In: Ecotoxicology, vol. 13, issue 5, pp 377–398. https://doi.org/10.1023/B:ECTX.0000035290.89590.03
Qayoom U, Bhat SU, Ahmad I, Kumar A (2022) Assessment of potential risks of heavy metals from wastewater treatment plants of Srinagar city, Kashmir. Int J Environ Sci Technol 19(3):9027–9046. https://doi.org/10.1007/s13762-021-03612-8
Radić S, Stipaničev D, Cvjetko P, Rajčić MM, Širac S, Pevalek-Kozlina B, Pavlica M (2011) Duckweed Lemna minor as a tool for testing toxicity and genotoxicity of surface waters. Elsevier 74(2):182–187. https://doi.org/10.1016/j.ecoenv.2010.06.011
Ritz C (2010) Toward a unified approach to dose-response modeling in ecotoxicology. Environ Toxicol Chem 29(1):220–229. https://doi.org/10.1002/ETC.7
Ritz C, Streibig JC (2005) Bioassay analysis using R. J Stat Softw 12(1):1–22. https://doi.org/10.18637/JSS.V012.I05
Rodrigues S, Pinto I, Martins F, Formigo N, Antunes SC (2021) Can biochemical endpoints improve the sensitivity of the biomonitoring strategy using bioassays with standard species, for water quality evaluation? Ecotoxicol Environ Saf 215:112151. https://doi.org/10.1016/J.ECOENV.2021.112151
Safitri R, Priadie B, Miranti M, Astuti A (2015) Ability of bacterial consortium: Bacillus coagulans, Bacilus licheniformis, Bacillus pumilus, Bacillus subtilis, Nitrosomonas sp. and Pseudomonas putida in bioremediation of wastewater in cisirung wastewater treatment plant. AgroLife Sc J 4(4):146–152
Schwartz W (1975) Janet R. Stein (Editor), Handbook of phycological methods, culture methods and growth measurements. XII, 448 S., 52 Abb., 44 Tab. Cambridge 1973: University Press, Ł 8. Z Allg Mikrobiol. https://doi.org/10.1002/jobm.19750150322
Serra T, Soler M, Pous N (2019) Daphnia magna filtration, swimming and mortality under ammonium, nitrite, nitrate and phosphate. Sci Total Environ 656:331–337. https://doi.org/10.1016/j.scitotenv.2018.11.382
Shiny KJ, Remani KN, Nirmala E (2005) Biotreatment of wastewater using aquatic invertebrates, Daphnia magna and Paramecium caudatum. Biores Technol 96(1):55–58. https://doi.org/10.1016/j.biortech.2004.01.008
Singh VK, Singh J (2006) Toxicity of industrial wastewater to the aquatic plant Lemna minor L. J Environ Biol 37(2), 385–390. http://jeb.co.in/journal_issues/200604_apr06_supp/paper_14.pdf
Smitha H, Raghavendra M, Shruthi S, Girishi K (2012) Bioremediation of rubber processing industry effluent by Arthrobacter sp. Int J Res Environ Sci Technol 2(2):31–34
Sonune NA, Garode AM (2015) Bioremediation potential of bacterial isolates for municipal wastewater treatment. Curr World Environ 10(2):619–625. https://doi.org/10.12944/CWE.10.2.27
Sturve J, Almroth BC, Förlin L (2008) Oxidative stress in rainbow trout (Oncorhynchus mykiss) exposed to sewage treatment plant effluent. Ecotoxicol Environ Saf 70(3):446–452. https://doi.org/10.1016/J.ECOENV.2007.12.004
Taraldsen JE, Norberg-King TJ (1990) New method for determining effluent toxicity using duckweed (Lemna minor). Environ Toxicol Chem 9(6):761–767. https://doi.org/10.1002/ETC.5620090610
Vasquez MI, Fatta-Kassinos D (2013) Is the evaluation of ‘traditional’ physicochemical parameters sufficient to explain the potential toxicity of the treated wastewater at sewage treatment plants? Environ Sci Pollut Res 20(6):3516–3528. https://doi.org/10.1007/s11356-013-1637-6
Williamson CE, Morris DP, Pace ML, Olson OG (1999) Dissolved organic carbon and nutrients as regulators of lake ecosystems: resurrection of a more integrated paradigm. Limnol Oceanogr 44(3):795–803. https://doi.org/10.4319/lo.1999.44.3_part_2.0795
Yang J (2013) Membrane bioreactor for wastewater treatment. The Ebook Company, Bookboon. http://125.234.102.27/handle/TVDHBRVT/15404
Yi X, Kang SW, Jung J (2010) Long-term evaluation of lethal and sublethal toxicity of industrial effluents using Daphnia magna and Moina macrocopa. J Hazard Mater 178(3):982–987. https://doi.org/10.1016/j.jhazmat.2010.02.034
Zaltauskaite J, Sujetoviene G, Cypaite A (2011) Municipal effluents toxicity evaluation using higher terrestrial, aquatic plants, and invertebrates. Environ Eng 2:713–718
Zaltauskaite J, Sujetoviene G, Cypaite A, Auzbikaviciute A (2014) Lemna minor as a tool for wastewater toxicity assessment and pollutants removal agent. Environ Eng. Proc Int Confer Environ Eng. https://doi.org/10.3846/enviro.2014.104
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by National Funds (through the FCT—Foundation for Science and Technology) and the strategic program UIDB/04423/2020 and UIDP/04423/2020. Bárbara Diogo is supported by a PhD grant (ref. 2022.10505.BD). Sara Rodrigues and Sara Antunes are hired through the Regulamento do Emprego Científico e Tecnológico—RJEC from the Portuguese Foundation for Science and Technology (FCT) program (2020.00464.CEECIND and CEECIND/01756/2017, respectively).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by Bárbara Diogo, Sara Rodrigues, and Sara Antunes. The first draft of the manuscript was written by Bárbara Diogo. Sara Rodrigues, Olga Lage, and Sara Antunes assisted during the elaboration of article/statistical analyses. All authors discussed the results, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent for publication
All of the authors have read and approved the final version to submit the paper and guarantee that it has not been published previously.
Consent to participate
This work is approved by all authors and by the responsible authorities where the work was carried out.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diogo, B.S., Rodrigues, S., Lage, O.M. et al. Are the ecotoxicological tools viable to evaluate the effectiveness of wastewater treatment plant effluents?. Int. J. Environ. Sci. Technol. 20, 11943–11962 (2023). https://doi.org/10.1007/s13762-023-04791-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04791-2