Abstract
Surface casing vents divert natural gas migration along oil and gas boreholes to bypass groundwater, with the gas venting to the atmosphere. While this strategy is designed to protect groundwater, it constitutes a source of greenhouse gases to the atmosphere. In instances where gas leakage occurs, the characterization of the molecular and isotopic composition of natural gas emitted from surface casing vent flows can be used to assist in identifying the gas source. We compare concentration measurements of non-hydrocarbon gases (within natural gas) of samples analyzed by laboratory-based gas chromatography (N2, Ar, CO2 and O2) and magnetic sector noble gas mass spectrometry (He, Ar and Kr) with field measurements conducted using a field portable quadrupole mass spectrometer (miniRUEDI). The standard deviation of miniRUEDI concentration results was within plus/minus one standard deviation of samples measured using laboratory-based GC (N2, O2, Ar and He) and magnetic sector noble gas mass spectrometry (He, Ar). Additional laboratory-based determination of isotope ratios of methane and argon (δ13CCH4, δ2HCH4, and 40Ar/36Ar) enabled a comparison between information provided by the analysis of reactive gases compared with noble gas isotopes. Gases from different sources displayed quantifiable differences in δ13CCH4 and δ2HCH4, but these changes may or may not be distinguished if only one sampling event is conducted. By comparison, 40Ar/36Ar further enabled the differentiation of various gas sources. The objective of this paper is to discuss the advantages and trade-offs of the three different analysis methods considered, and the feasibility of their application in different environmental monitoring scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Well integrity is the application of “technical, operational and organizational solutions to reduce the risk of uncontrolled [and unintended] release of formation fluids throughout the life cycle of a well” (Standards Norway 2013). Saline formation water, crude oil and/or natural gas migration can occur either as buoyant single phase flow or by transport phenomena such as advection and diffusion through variably porous and permeable media. These fluid transport phenomena occur naturally in sedimentary basins, but also can be facilitated anthropogenically along some well bores that experience wellbore “integrity” or isolation issues (Dusseault and Jackson 2014). These anthropogenically induced fluid flow events are commonly termed fugitive or stray gas events and are among the most commonly reported groundwater contamination events associated with shale gas drilling (Darrah 2018; Darrah et al. 2014). These fugitive gas release events occur most commonly as fluid flow within the typically cement-filled well annulus outside the well casing and potentially result in formation water or petroleum and/or fugitive gas migration into overlying casing strings, rock successions, surface environments or the atmosphere. The effects of these fugitive releases include aquifer contamination (Darrah et al. 2014, 2015a; Jackson et al. 2013; Kelly et al. 1985) or greenhouse gas (GHG) emissions (Wigston et al. 2019). Well integrity issues can result in either surface casing vent flow (SCVF), where fluids migrate intentionally from inside the surface casing, or gas migration (GM), where fluids migrate locally outside the commonly cement-filled wellbore and into adjacent formations, aquifers or soils (Watson 2004; Watson and Bachu 2009; Wigston et al. 2019; Wisen et al. 2020).
It is common to characterize the chemical composition of gases produced from wells and surface casing vents (SCV) to understand the source of gases. Such characterizing may include both hydrocarbon and non-hydrocarbon gases (He, N2, Ar, CO2 and O2, and Kr). The objective of this study is to compare three analytical methods used to characterize non-hydrocarbon gases and as well as insights that can be gained from analysis of these gases and their isotopes in comparison with isotopes of δ2H and δ13C from CH4. This analysis is conducted on samples collected from a site located near Brooks, Alberta, Canada from January 2018 to January 2020.
Background
Surface casing vents capture and divert gas from a wellbore through shallow formations (SCV, Fig. 1). SCV are an important well integrity technology that permits gases, most commonly natural gas, to vent atmospherically from within the surface casing annulus and the deeper, commonly cement-filled, wellbore annulus. The purpose of a SCV is to improve wellbore integrity, most commonly by reducing material and environmental risks associated with annular casing pressure (ACP), also termed annular buildup pressure (ABP), that results from a gas column collecting in the surface casing or wellbore annulus. Provided that the SCV flow (SCVF) rate and gauge pressure are sufficiently low, a SCV improves wellbore integrity and eliminates or reduces impacts via gas migration on soil and groundwater resources, but emits GHG’s to the atmosphere. In Alberta, a SCV is defined as having a flow (SCVF) if it fails a bubble test; where a hose is connected to the SCV and placed under 2.5 cm of water for 10 min (Alberta Energy Regulator 2021a). If any bubbles come from the tube, then the well has a SCVF. Serious SCVF’s are categorized by the impacts of the gas released, due to its rate, association with gas migration beyond the well annulus, or the gas composition (Alberta Energy Regulator 2021a). Wells with serious SCVF are subject to remediation to render the SCVF or other gas migration effects at or near these wells to either non-emitting, or non-serious status, as required by regulations (Alberta Energy Regulator 2021b). As of June 2, 2016, 10,326 Alberta petroleum wells had SCVF (Wigston et al. 2019). Among these wells, 337 had serious SCVF’s, with total annual emissions of 24.9 × 106 m3 of natural gas, composed of predominantly methane. There were 9,989 wells with non-serious SCVF in Alberta, typically “sweet” (low sulfur) gas emissions ≤ 300 m3/day (Wigston et al. 2019). It is estimated that these non-serious SCVF’s produce 59.5 × 106 m3/yr of natural gas that is emitted to the atmosphere.
However, these wellbore integrity failures affect a small fraction of petroleum wells (in 2016, 5% of well drilled in Alberta developed leakage) and many wellbore integrity risks are significantly reduced by improved well design and construction practise (Wigston et al. 2019). For example, in Alberta, Canada, the practise of “cementing casing to surface” reduced the incidence of SCVF from 11.5 to 3.9% of petroleum wells drilled, although the same device produced no effective reduction in the albeit small, occurrence rate of gas migration, which were 0.7 and 0.6%, respectively (Boyer 2016a, b). Specific, although small, subsets of petroleum wells, particularly inclined (i.e., not drilled vertically) petroleum wells are associated with higher wellbore integrity risks (Hardie and Lewis 2015; Bachu 2017). Nonetheless, gas migration and aquifer contamination remain rare events (Tilley and Muehlenbachs 2012; Wigston et al. 2019).
As serious SCVF must be remediated, only wells with non-serious SCVF can leak for an extended period of time. The average daily rate of gas emitted from wells with non-serious SCVF is 13.2 m3/day/well. Improved well design and construction reduce, but do not eliminate, the rate at which newly constructed wells have both serious and non-serious SCVFs (Wigston et al. 2019).
In Canada and throughout the US public concern has focused on natural gas migration in the immediate vicinity of petroleum wells, often in association with either well construction, well stimulation (commonly by hydraulic fracturing), or fluid injection and storage, as well as the potential for aquifer contamination (Cherry et al. 2014; Darrah et al. 2014; Jackson et al. 2011, 2014; Romanak et al. 2013; Summers 2010; Vengosh et al. 2014). In Alberta, water well owners expressed high levels of concern about the potential for CH4 in groundwater, but rated CH4 in their well water as their most infrequent water quality issue (Summers 2010). However there have haven relatively few (likely on the order of a few dozen) proven and alleged cases of aquifer contamination due to gas migration associated with oil and gas wells and the lack of documented aquifer contamination cases suggest that this effect is rare compared to the number of cases of near wellbore natural gas migration (Wigston et al. 2019). The documented effects of natural gas seepages (Noomen et al. 2012; Harkness et al. 2017; Kreuzer et al. 2018), a gas well blow out (Brantley et al. 2014; Darrah et al. 2014; Kelly et al. 1985) and gas main leakages (Phillips et al. 2013; Jackson et al. 2013) can produce similar geochemical and biological effects and impacts as those associated with well integrity-caused gas migration and aquifer contamination (Goodwin et al. 1990; Szatkowski et al. 2002; Van Stempvoort et al. 2005; Noomen et al. 2012; Tilley and Muehlenbachs, 2012). The recognition of aquifer and groundwater contamination by natural gas migration is rendered more difficult in locations where the natural occurrence of methane in groundwater is common (Cheung and Mayer, 2009; Humez et al. 2016a; 2016b; 2016c; Mayer et al. 2015; Hendry et al. 2016; 2017).
Recent research has focused on understanding specific geochemical and hydrogeological aspects of natural gas migration, from well bore integrity (Wigston et al. 2019), to the study of the migration and fate of injected gas in shallow aquifers (Forde et al. 2019; Cahill et al. 2017; 2018; 2019). While repairing a petroleum well with SCVF remains an important engineering challenge (Watson 2004; Watson and Bachu 2009; Dusseault and Jackson 2014; Dusseault et al. 2014), geochemical methods (Tilley and Muehlenbachs 2012; Darrah et al. 2014; Jackson et al. 2013; Mayer et al. 2015; McIntosh et al. 2019) can be used along with wireline well logging methods (Aslanyan et al. 2015) to identify the depth at which natural gas enters the well annulus.
Subsurface gas composition
The ultimate source of SCVF emissions is subsurface natural gas resources. While methane is the predominant component of natural gas, there is considerable variability in the concentrations of methane, higher alkanes and other constituents dependent on the maturity and history of the reservoir (Hitcheon 1963a; b; c; James and Burns 1984). Dry gas contains > 95% methane in the hydrocarbon components, while wet gas contains 5 to 95% of higher molecular weight, non-methane aliphatics, but contains a greater proportion of heavy hydrocarbons (Cody and Hutcheon 1994). Primary biogenic gas is expected to be composed of almost exclusively methane, while thermogenic gases contain varying concentrations of other heavier hydrocarbons including ethane, propane and others (e.g., Stolper et al. 2015). Primary biogenic and thermogenic gases typically have different δ13CCH4 and δ2HCH4 values, with biogenic gas typically having lower δ13C values than thermogenic gas (Schoell 1983; Clark and Fritz 1997). Gas from SCVF is typically composed of > 90% methane (Wigston et al. 2019). Characterization of the gas composition and isotopic composition of methane and higher alkanes has been used to estimate the depth where gas enters the petroleum well bore annulus (Szatkowski et al. 2002; Tilley and Muehlenbachs 2012; Mayer et al. 2015; Sandau 2016a, b; McIntosh et al. 2019). While such geochemical and isotopic “fingerprinting” approaches of hydrocarbons have been used successfully in some cases, both the potential for compositional and isotopic alteration by microbial biodegradation or oxidation of samples post-sampling (Tilley and Muehlenbachs 2006; Vinson et al. 2017) or a lack of baseline studies that define the original geochemistry (Tilley and Muehlenbachs 2006; Mayer 2015; Hendry et al. 2016; 2017; Darrah 2018) often compromise the use of such methods . Therefore, additional tracer techniques are desirable.
Noble gases
The noble gases (helium, neon, argon, krypton and xenon), which are present as as minor to trace constituents of natural gases (Hitcheon 1963c; Hutcheon 1999) can be used as tracers to compliment the chemical and isotopic composition of hydrocarbon gases (Hunt et al. 2012; Darrah et al. 2014). Noble gases are inert and therefore not susceptible to biodegradation. These chemical characteristics make them potentially useful tracers in natural gases. In the subsurface, noble gases are primarily sourced from three reservoirs, the (1) atmospheric (air or air-saturated water that dominates the composition of noble gases in the hydrosphere), (2) crustal noble gases, including radiogenic and nucleogenic noble gases produced either directly or indirectly by radioactive decay, and (3) mantle-derived noble gases (Ballentine et al. 2002). Atmospheric noble gases are transported into the subsurface via groundwater recharge. Within the Earth, radioactive decay of elements in crustal minerals results in the production of noble gases such of 4He (directly) and 21Ne (indirectly) from the decay of U and Th, while 40Ar is produced by the decay of K. In comparison, primordial 3He trapped since the formation of the Earth can be released by magmatic processes and used to quantify mantle contributions. In the continental crust, the composition of noble gases, specifically in groundwater, are predominantly a mixture of atmospheric and crustal gases. Young groundwater recharged since the 1960’s will contain additional 3He due to the decay of 3H sourced from the atmospheric testing of thermonuclear weapons (e.g., Clark and Fritz 1997; Utting et al. 2012; Palcsu et al. 2017). The accumulation of other noble gases and their isotope ratios can also be used for dating water of different residence times (Ballentine et al. 2002).
In addition to the accumulation of tritiogenic 3He and crustal noble gases due to radioactive decay, noble gas composition may be further affected by physical processes such as mixing, degassing, and re-dissolution (Darrah et al. 2014, 2015b). As a result, in addition to residence time calculations, noble gases can be used as an indicator of mixing between atmospheric and crustal components (Castro et al. 1998) and reveal the mechanisms of fluid transport, specifically within groundwater.
Hiyagon and Kennedy (1992) previously sampled hydrocarbon-containing gases from petroleum wells producing from Cretaceous and Devonian reservoirs in Alberta for noble gas analysis. They subdivided their samples into two noble gas compositional groups: Group A samples were sourced from shallower depths in the crust from wells located in the southern part of Alberta, and had noble gas compositions indigenous to the sediments with anomalously higher 3He/4He ratios. Group B samples were sampled from deeper gas pools located in the northern part of Alberta with a composition consistent with noble gas production from the continental crust that underlies the Phanerozoic sedimentary basin.
An important component of obtaining robust noble gas data is the verification of appropriate sample collection and sample handling techniques. The standard best practice method for the collection of noble gas samples involves the collection of water or gas samples using refrigeration-grade copper tubes which are crimped or clamped closed. Once returned to the laboratory, gas samples are purified to remove reactive non-noble gases using a series of traps and getters described in detail elsewhere (Darrah and Poreda 2012; Heilweil et al. 2016; Moore et al. 2020). Depending on the design of the noble gas line and the elements of interest, the purified gas is then analyzed using either a magnetic sector or quadrupole mass spectrometer, or combinations thereof. While this method produces reliable results and allows for the measurement of many noble gas isotopes, laboratory-based analysis is relatively costly and time consuming. Laboratory-based noble gas analyses are also limited by the relatively low number of laboratory facilities due to the costly laboratory sample preparation lines and instrumentation required. For example, the establishment of a modern sample preparation line and magnetic sector mass spectrometer costs between $550 and 850 K USD, depending on the set up, instrument manufacturers, and intended use. Further, noble gas measurements by this approach are time consuming and typically require 2–4 h of analytical time per sample. Globally there are relatively few noble gas laboratories, with even a more limited number of laboratories that conduct external commercial analysis. Analytical commercial rates are variable, ranging from $300 to over $1000 USD per sample depending on the sample type, the number of isotopes of interest, and the required precision.
Alternatively, other methods can be used to measure noble gas concentrations. Depending on instrumentation and concentrations of target compounds, some noble gases (e.g., He, Ar, Kr, and Xe) can be readily measured using gas chromatography (GC). Another method to measure noble gas concentrations uses a quadrupole mass spectrometer (or residual gas analyzers), which are often used in tandem with sector magnet mass spectrometers in the laboratory or independently as a field portable unit. For example, the miniRUEDI uses a quadrupole mass spectrometer (i.e., quadrupole mass analyzer or residual gas analyzer) to measure the concentrations of select reactive and noble gases (Brennwald et al. 2016). Depending on the matrix, the miniRUEDI can be used to measure the concentrations of He, Ne, Ar, Kr, N2, O2 and CO2 with uncertainty on the range of 1 to 3% of the respective analytes.
Objectives of this study
As previously described, the objective of this study is to provide a better understanding of the advantages and disadvantages of different analytical approaches for measuring non-hydrocarbon gases: He, N2, Ar, CO2 and O2, and Kr to enhance planning and capabilities of future field sampling campaigns. The method or combination of methods that best meet the analytical needs of the research project will depend on the goals of the study, field work constraints, sample access constraints, and budgetary considerations. These different analytical approaches were compared by analyzing samples from surface casing vent flows and a well annulus. The goals of this study are to compare different methods of measuring gas concentrations, as well as to investigate compositional and isotopic variability over time.
This was achieved by comparing the composition and isotopic ratios of natural gas samples collected in the field and analyzed using three different analytical approaches (i.e., three different types of instruments). These instruments include:
-
1.
Lab based gas chromatography analysis of samples collected in standard glass GC vials with butyl septa;
-
2.
Lab based noble gas magnetic sector mass spectrometer analysis of samples collected using copper tubes;
-
3.
miniRUEDI analysis of samples aliquoted directly from fluid streams in the field.
Materials and methods
Description of location and wells
Samples were collected at the CMC Research Institutes Inc. Countess Field Research Station (FRS). The FRS is located in Newell County, about 200 km southeast of Calgary, Alberta (Fig. 2). At the FRS, a CO2 Injection well has been completed at a depth of 300 m in the Brosseau Member of the Late Cretaceous Foremost Formation. Approximately 20 m to the southeast of the Injection well is a Geophysical observation well, and approximately 20 m to the north of the Injection well is a Geochemistry observation well. The Geophysical monitoring well is instrumented to 300 m depth with a variety of geophysical equipment. The Geochemistry Observation well production casing is open to the formation at about 300 m depth through screens in the production casing. The Injection well was drilled in March 2015, and the Geochemistry and Geophysics observation wells were drilled in February–March 2016.
Each well has surface casing that extends to depths of: 68 m below ground surface (bgs) for the Geophysics observation well, 120 m bgs for the Geochemistry observation well and 226 m bgs for the Injection well. The ground elevation of all three wells is essentially the same. The surface casing at all three wells is vented to the atmosphere via a Surface Casing Vent (SCV) when the SCV valve is open (Fig. 1). The SCV of these three wells emits gas at different rates.
Field methods
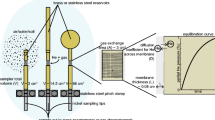
Prior to collection of gases, SCV valves were closed to allow gas to accumulate in sufficient quantity to permit for purging of well plumbing and sampling equipment prior to sample collection. Tubing was connected to the SCV to allow gas to be directed into sample glass GC vials, refrigeration-grade copper tubes and the miniRUEDI field mass spectrometer (Fig. 3). Prior to sample collection gas from the SCV was flowed through tubing for at least one minute (> 5 volumes of the tubing) to purge the tubing.
Vials for standard GC analyses were filled with SCVF gas using a water-filled bucket that was placed at the end of the tubing to provide a water trap that isolated the sample from backflow of atmospheric “air” contamination (Fig. 3). Gas for GC analysis was collected in a water-filled syringe inverted in the water trap to allow for displacement of water with gas. The gas was then injected into a 125 mL glass septum bottles with butyl rubber stoppers. Prior to collection, bottles were treated with mercuric chloride to prevent microbial sample alteration and evacuated to a pressure of ~ 10–3 mbar. Samples collected for magnetic sector mass spectroscopy were collected from the same sampling apparatus by attaching a 3/8-inch refrigeration-grade copper tube inline and then sealing by cold-welding with brass refrigeration clamps. For the direct measurement of samples using the miniRUEDI in the field, the same tubing from the SCV was connected to a tubing with smaller diameter then connected directly to the mass spectrometer.
Three of the sample sources were surface casing vents and one was a well annulus. The study period presented in this manuscript is from January 2018 to January 2020. Over this period the gas flow varied from the different gas sources and samples could not be obtained from each source during each sampling event. The Geophysics observation well SCV has continually produced gas during the course of the sampling period with gas samples being collected on 12 occasions. The Injection SCV started to produce gas in the summer of 2018, with the first sample collected for this study in August 2018 and gas samples being collected on 8 occasions. Initially the Geochemistry Observation well SCV and the Geochemistry Observation well production casing annulus produced gas. To clear the well of bio-fouling, a highly saline (KCl) fluid was added to the well in December 2018 to suppress ongoing microbial natural gas production. Since December 2018 insufficient gas was produced to be able to collect a sample from the Geochemistry Observation well SCV and Geochemistry Observation well production casing annulus. The Geochemistry Observation well production casing annulus has been sampled 5 times and Geochemistry Observation well SCV has been sampled 9 times.
Analytical methods
Gas chromatograph (GC)
Determination of the gas compositions by GC analysis was conducted at the University of Calgary and the University of Ottawa. Most samples were analyzed using a Scion 450/456 gas chromatograph (GC) using argon and hydrogen carrier gases (hydrogen used when measuring argon, and vice versa). The GC is a four channel GC and includes four separate injection loops, four separate analytical columns (a Molsieve 13 × 45/60 (12’ × 1/8″) packed analytical column; a Haysep N 80/100 Mesh (3’ × 1/8″) packed analytical column; a CP-Sil 5CB analytical column (10 m × 0.15 mm, 2 µm film thickness), and a MXT-Molsieve 5A analytical column (30 m × 0.53 mm, 50 µm film thickness)), three thermal conductivity detectors and a flame-ionization detector for gas separation and quantification. Gas analyses were conducted by injection of 5 mL aliquots of each gas sample and the detection limit for non-hydrocarbon gases was 50 ppm, while the detection limit for alkanes including methane was 0.5 ppm. Certified gas standards were used to calibrate the GC prior to the analyses. Analytical precision and accuracy for gas composition analysis is better than ± 2.5% of the reported concentrations. Two samples were analyzed at the University of Ottawa Ján Veizer Stable Isotope Laboratory using an SRI GC 8610C with helium as a carrier gas.
Magnetic sector mass spectrometry (MSMS) noble gas measurements
Full noble gases analysis was conducted at the WHEEL Noble Gas Laboratory at Ohio State University. The method is described in detail in several prior publications (Darrah and Poreda, 2012; Kang et al. 2016; Moore et al. 2020). Briefly, after returning to the laboratory, samples were attached to the ultra-high vacuum line and an aliquot was introduced for sequential measurement by in-laboratory quadrupole mass spectrometry (SRS Residual Gas Analyzer 200) for quantification of major (e.g., CH4, CO2, N2, Ar) and noble gases (e.g., He, Ar, Kr). Separate aliquots were then prepared for magnetic sector mass spectrometry by sequential use of getters to remove active gases and achieve cryogenic separation. Noble gas concentrations were determined by comparison to the in-house Lake Erie Air reference material (Kang et al. 2016) and the Scripps Institute of Oceanography Yellowstone Murdering Mudpots standards reference material (Darrah and Poreda 2012). Uncertainties for noble gas measurements as part of this analyses are denoted in parentheses: 4He (0.91%), 22Ne (1.44%), 36Ar (0.68%), 84Kr (1.89%), and 132Xe (2.29%). Helium isotopic errors averaged ± 0.0091 times the ratio of air (or 1.26 × 10–8) for 3He/4He as determined by measuring the atmospheric air reference material (Kang et al. 2016) and Scripps Institute of Oceanography MM helium standard (Darrah and Poreda, 2012). Neon isotope ratios were corrected for interference by 40Ar2+ and CO22+ (40Ar2+ is typically < 10% of total 20Ne signal on the Faraday cup and CO22+ is typically < 2.5% of the total 22Ne signal on the Faraday cup) (Darrah and Poreda, 2012). The neon isotopic errors were < ± 0.46% for 20Ne/22Ne and < ± 0.87% for 21Ne/22Ne, respectively, as determined by measuring the atmospheric air reference material. The argon isotopic errors were < ± 0.83% for 38Ar/36Ar and < ± 0.65% for 40Ar/36Ar, respectively, as determined by measuring the atmospheric air reference material.
miniRUEDI
The miniRUEDI is a field portable mass spectrometry system that fits in a plastic box that is 84 cm × 48 cm × 31 cm. To achieve vacuum, the system uses a KNF diaphragm vacuum pump and Pfeiffer HiPace80 turbo pump. The system uses a SRS residual gas analyzer 200 quadrupole mass spectrometer to measure concentrations of N2, O2, He, Ar and Kr. For calibration of N2, O2, He, Ar and Kr the system uses atmospheric air from the field as the standard; other gases can be analyzed but require a separate standard. Air standards are run between each sample. Measurement and data calculation are conducted using the system software. The system is described in full in Brennwald et al. 2016. An analysis of the concentrations of the range of gases selected (N2, O2, He, Ar and Kr) takes about 10 min, an air standard is run before and after each analysis, as such to run the analysis in full is approximately 30 min. The limits of detection of the instrument are estimated at 1% of the concentrations in atmospheric air (Brennwald et al. 2016).
Stable isotope composition of methane (δ 13CCH4 and δ 2HCH4)
The majority of isotope ratio measurements on methane were conducted at the University of Calgary, while two samples were analyzed at the University of Ottawa. Carbon and hydrogen isotope ratios (δ13CCH4 and δ2HCH4) were measured at the University of Calgary using a Thermo Trace GC – GC–IsoLink system interfaced to a Thermo 253 mass spectrometer via a Thermo Conflo IV. 13C/12C ratios are reported in the internationally accepted delta notation compared to the Vienna Pee Dee Belemnite (VPDB) standard, and 2H/1H results are reported compared to the Vienna Standard Mean Ocean Water (V-SMOW) standard. Two samples were measured at the University of Ottawa Ján Veizer Stable Isotope Laboratory for the isotopic composition of methane using a Thermo Scientific Delta V Isotope Ratio Mass Spectrometer (Hudson 2004; Kampbell et al. 1998; Thermo Fisher Scientific 2011). Measurement uncertainties were better than 0.5 ‰ for δ13CCH4 and 3.0 ‰ for δ2HCH4.
Results and discussion
Gas chemistry
The results of the geochemical analysis of SCV gas samples conducted using GC, miniRUEDI, and MSMS, as well as isotopic analyses of CH4 are summarized in Table 1. Results are listed for the three SCV and one well annulus source sampled consecutively over time. Different types of samples have been collected at different times. From the four sample sources the concentration of N2 ranged from 0.02 to 0.82 cm3STP/cm3, for O2 ranged from 0.001 to 0.21 cm3STP/cm3, for CH4 ranged from 0.11 to 0.98 cm3STP/cm3, for CO2 ranged from 4.90 × 10–5 to 8.01 × 10–3 cm3STP/cm3, for He ranged from 2.6 × 10–5 to 1.0 × 10–3 cm3STP/cm3, for Ar ranged from 2.6 × 10–4 to 1.0 × 10–2 cm3STP/cm3, for Kr ranged from 7.01 × 10–8 to 6.22 × 10–6 cm3STP/cm3. The [(3He/4Hesample)/ (3He/4Heair)] (R/RA) ranged from 0.09 to 0.37 and the 40Ar /36Ar ranged from 295 to 319. The δ13CCH4 ranged from − 71.7 to − 53.8 ‰ nd the δ2HCH4 varied from − 291 to − 258 ‰.
Comparison of analysis methods
For a comparison of gas concentrations obtained by different analytical methods, we have made the underlying assumption that the gas sources are not changing over time, the validity of this assumption will be discussed later in the section Time Series. The gas concentrations measured with the three methods are shown in Fig. 4a–e. To compare the results, the averages of all samples from a given gas source were calculated, and the standard deviation was determined.
Concentrations of gases measured using GC, miniRUEDI and magnetic sector mass spectrometer (MSMS) for: a nitrogen, b oxygen, c argon, d helium and e) krypton. Averages are shown in filled circles with error bars shown, while individual measurements are shown as open circles. Geochem annulus = Geochemistry Observation well production casing annulus; Geochem SCV = Geochemistry Observation well SCV; Geophysical SCV = Geophysics observation well SCV; and Injection SCV = Injection well SCV
mini-Ruedi vs. GC
N2, O2, Ar and He were all measured using both GC and miniRUEDI for at least some samples (N2: 4 samples, O2: 4 samples, Ar: 3 samples, He: 2 samples, Table 1, Fig. 4a–e). When comparing the results from the GC and miniRUEDI for N2, O2, Ar and He concentrations the average plus/minus the standard deviation overlapped.
In addition to comparing SCV gas concentration results based on the range of one standard deviation surrounding the average, linear regressions were conducted for each compound/element measured. Linear regressions were conducted using 20 samples for N2 and O2, 10 samples for Ar and 5 samples for He. For this analysis, a comparison was conducted between samples collected on the day miniRUEDI analysis was performed (Fig. 5). For each compound/element a linear regression trend line was calculated and forced through zero, as it is assumed that both methods should record below detection limit when no gas is present. For N2 the regression between samples measured on the GC and samples measured in the field using the miniRUEDI had a slope of 1.30, and an R2 of 0.60. N2 concentrations tended to be higher when measured on the miniRUEDI compared to GC measurements. For Ar the regression has a slope of 1.2 and a R2 of 0.67. For O2 the regression has a slope of 1.24 and a R2 of 0.27. Helium was only measured for some samples using GC and has a R2 of 0.2. All the gases measured on both the miniRUEDI and GC (N2, O2, He and Ar) have a regression slope greater than 1. On the regressions shown in Fig. 5 the samples with higher concentrations measured by the miniRUEDI compared to the GC were often at elevated concentrations. It is possible that these higher concentrations are the result of slight air contamination compared to the GC samples. With the configuration used in these experiments CO2 and CH4 concentrations were not measured. CH4 represents a large proportion of most of these samples, and as such gas concentrations could not be normalized based on total gas concentration.
In general the concentrations measured using the miniRUEDI produce results where the average of multiple results from the same feature is within the error of multiple samples from the same feature measured by GC analysis. Each analysis method is distinct and has different sources of error. The miniRUEDI uses only one calibration volume and in the configuration used does not have the resolution to differentiate gases with nearly same mass, such as O2 (molar mass 15.99) and CH4 (molar mass 16.04) as is possible with a GC. However, the gas is injected directly into the miniRUEDI from the gas source, limiting possibilities of handling issues with the exception of potential air contamination should fittings between the well and the instrument leak. In comparison, samples collected for GC analysis undergo significant shipping and handling during the sampling, transport, and analytical procedures. Moreover, samples collected for GC analyses are commonly stored longer prior to their analysis. This compendium of factors provide greater potential for the post-sampling alteration of samples prior to analysis by GC. Both samples analyzed by miniRUEDI and by GC have the potential to be contaminated with air during sampling. With the miniRUEDI, if gas is not sufficiently purged or a valve connection is not tight, there is additional potential for air leakage. With glass vials for GC analysis there is potential for air to contaminate the vial during injection via syringe or from a loose connection on the instrument. Both methods are subject to human error that can be greater in adverse conditions that may exist during field sampling.
miniRUEDI vs. magnetic sector mass spectrometer
The noble gas concentrations measured in SCV gases using the miniRUEDI were also compared with results determined using a magnetic sector mass spectrometer (MSMS). Noble gas measurements conducted using MSMS typically allow for fewer replicates and/or fewer total samples to be measured as part of a typical field study. Gas concentrations measured with both methods are shown in Fig. 4c–e. Results are compared based on the range of one standard deviation surrounding the average. When the average plus/minus one standard deviation is compared, the concentrations of Ar and He are within the same range, which is similar to the results obtained for the miniRUEDI/GC comparison. With both methods, the concentration of Ar from the Geochemistry observation well production casing annulus and the Geochemistry observation well SCV tend to be close to the concentration for atmospheric air, while the Geophysics observation well SCV had lower Ar concentrations, consistent with a deeper gas source. Helium concentrations measured with both methods for the Geochemistry observation well production casing annulus and the Geochemistry Observation well SCV are also much lower than for the Geophysics observation well SCV, also consistent with a deeper geogenic gas source that has intrinsically higher ratios of gas to water. For Kr, only one sample was analyzed from the Geochemistry observation well production casing annulus using MSMS so no standard deviation could be calculated. For the samples from Geochemistry observation well SCV and Geophysics observation well SCV there is overlap between the averages measured plus/minus one standard deviation. The samples with more replicates show considerable variability of Kr concentrations for both methods.
Comparing methods for gas analysis
The three methods used during this study to measure gas concentrations of SCV and well annulus samples produced results where the average plus-minus one standard deviation of each method overlapped with the average plus minus one standard deviation of the other methods, with the exception of one Kr sample. Based on data from this study, we suggest that each analytical method has its own cohort of benefits and disadvantages. Some of these benefits and disadvantages are discussed below, with a summary provided in Table 2.
Sample collection for GC analysis is relatively simple in the field and the cost of the analysis is relatively low. GC analysis is the standard method for analysis of hydrocarbon gases present in gas samples. Furthermore, the ability to calibrate measurements repeatedly with certified standards is an additional plus for GCs operating under controlled lab conditions. While selected noble gas concentrations can be analyzed using GC, GC methods are not ideal approaches for measuring noble gas concentrations as the instrument is not under vacuum, resulting in a high potential for air contamination (e.g., specifically for components like argon). Furthermore, many GC systems use a noble gas (e.g., He or Ar) as a carrier gas meaning that gas cannot be measured in that configuration.
Collection of samples in copper tubes for noble gas analysis by MSMS can be done in a similar way to that for the collection of samples for GC analysis. This method has long been inferred to give the most reliable noble gas measurements (Weiss 1971a, b), as these samples go through numerous preparation stages (polished, refrigeration-grade copper and extensive purging) prior to their analysis. Once in the laboratory, vacuum preparation lines are also well calibrated for flexible measurement across large dynamic ranges. For example, various split factors can be used for delivery of appropriate quantities of sample gas to the MSMS following initial screening with quadrupole residual gas analyzers on the preparation line. As a result, the MSMS instrument is calibrated over different ranges and subsequently offers the best option for robust concentration and isotopic measurements of noble gases.
A major advantage of the miniRUEDI is that it allows results to be produced immediately in the field and minimizes risk associated with sample collection, transport, and sample preparation. As a result, this device can be deployed in various configurations to answer different research questions than can be answered by laboratory approaches in a time-sensitive fashion. For example, the system can be set up to conduct continuous monitoring of gas composition. The drawback of this instrument is that to reliably measure CH4 and CO2 concentrations additional calibrations are needed, and concentrations of higher order hydrocarbons (ethane, propane, etc.) cannot be reproducibly measured using this approach. While the instrument allows for quick measurement of gas concentrations, set up and running of the instrument take longer (approximately 50 min to analyze gas three times with each sample bracketed by a air standards) than the collection of a copper tube or glass vial. Still, this approach offers near-continuous sampling and rapid field-based assessment of gas composition.
For a given case study, the most appropriate analytical approach will depend on the site and the study objectives. In this study we have not deployed the miniRUEDI system to its full capacity, as air was used for calibration and hence the analysis was limited to concentrations of N2, O2, He, Ar and Kr. However if a different calibration gas was used it is possible to measure concentrations of CO2 and CH4 and potentially other gases. Additionally we have used the instrument solely for one-off measurements; the system could be kept at a site and connected to multiple SCV gas sampling sites enabling near continuous measurement of select gaseous compounds. The most effective use of the instrument is likely the pairing of the near-continuous measurements of select compounds with verified laboratory-validated measurements of the same and other compounds not accessible via miniRUEDI measurements. For example, the near-continuous miniRUEDI measurements for concentrations of N2, O2, He, Ar, Kr and potentially others could be paired with the noble gas isotopic measurements by MSMS noble gas systems. Similarly, the near-continuous miniRUEDI measurements for concentrations of N2, O2, He, Ar, Kr and potentially others can be paired with detailed hydrocarbon assessments offered by GC with flame ionization and thermal conductivity detection or either laboratory or field-based stable isotope measurements.
Time series
For a component of the comparison of methods it was assumed that the gas emanating from surface casing vents and well interior should be the same over time. This was assumed based on the fact that gas is sourced from a geologic source that is deep enough that it should not change seasonally. Over time the results from different methods, fall within the same range based on the previous discussion. However, is this assumption valid? To assess if this assumption is valid results time series of the gas results obtained from the current study are plotted in Fig. 6.
Time series of gas concentrations. MR indicates measurement using miniRUEDI, GC indicates measurement using gas chromatography, MSMS indicates magnetic sector mass spectrometer. a Ar, b He, c Kr, d N2 and e O2. Geochem annulus = Geochemistry Observation well production casing annulus; Geochem SCV = Geochemistry Observation well SCV; Geophysical SCV = Geophysics observation well SCV; and Injection SCV = Injection well SCV
The composition of gas from the Geophysics observation well SCV did not show consistent change over time (Fig. 6). Initially, the Injection SCV did not produce gas, and therefore was not sampled. The Injection SCV began producing gas in mid-2018. The first three samples collected in August, October and December 2018 showed greater variability in N2, O2 and Ar, and concentrations closer to atmospheric air than the samples collected in 2019. The variability is likely the result of flushing of atmospheric gases from the system and perhaps also that gas flow rates may have been lower initially leading to a greater potential mixture with gases derived from atmospheric sources (e.g., air-saturated water or air entrainment). The gas sourced from the Geochemistry observation well SCV and the Geochemistry observation well production casing annulus generally show a greater degree of variability in concentrations of N2, O2 and Ar than the gas from the Injection SCV and the Geophysics observation well SCV. This greater variability is attributed to the lower gas flow rates from these wells resulting in greater contamination with atmospheric air. It appears as though there was a slight trend toward increasing O2 and N2 concentrations in the Geochemistry observation well SCV, likely indicative of the decrease in flow of gases from this SCV with more atmospheric air mixing. When the samples from Geochemistry observation well SCV and the Geochemistry observation well production casing annulus are treated as one population, the calculated standard deviation tends to be higher than the standard deviation calculated from samples from Geophysics observation well SCV and the Injection SCV.
Based on the time series analysis it appears that the composition of gases did not show consistent changing trends over time, however variability was observed, which is most likely attributed to greater mixing with atmospheric air. This time series analysis appears to suggest that the assumption that gas composition did not change over the time of this analysis is valid, however it does not indicate if composition will or will not change in the future. It also indicates that over time there is viability from features sampled. This variability means that it is important to collect multiple samples over time to gain a better understanding of this variability to be able to detect a change related to a geologic source.
Isotopic compositions of methane, helium and argon
The δ13CCH4 vs. δ2HCH4 results are summarized in Table 1 and plotted in Fig. 7a. Multiple samples were collected over a two-year period. The samples from the Geochemistry observation well production casing annulus and the Geochemistry observation well SCV have methane of similar isotopic compositions (δ13CCH4 of -66.7 ± 2.7 and − 68.4 ± 2.0 ‰ δ2HCH4 of − 277 ± 3 and − 279 ± 7 ‰); the Geophysics observation well SCV and Injection well SCV had also similar isotopic compositions for methane to each other (δ13CCH4 of − 62.8 ± 3.7 and − 62.5 ± 0.9 ‰; δ2HCH4 of − 270 ± 8 and − 273 ± 2 ‰). When considering the averages plus/minus one standard deviation, there is a slight difference between the isotopic composition of methane of the Geochemistry observation well production casing annulus and the Geochemistry observation well SCV compared with the Geophysics observation well SCV and Injection well SCV. Based on the error observed from multiple measurements over time, if one sampling event was conducted it may be concluded that methane from both wells has the same isotopic composition, while many samples collected over time reveal they are in fact slightly different.
a δ2HCH4 vs. δ13CCH4 of methane in SCV samples and b R/RA vs. 40Ar/.36Ar of SCV samples. (Note: Full noble gas analysis not conducted on sample from Injection SCV). Geochem annulus = Geochemistry Observation well production casing annulus; Geochem SCV = Geochemistry Observation well SCV; and Geophysical SCV = Geophysics observation well SCV. Note: Hollow circles are individual samples, solid circles with black outline are sample averages
Figure 7b shows R/RA vs. 40Ar/36Ar of samples from Geochemistry Observation well production casing annulus, Geochemistry Observation well SCV and Geophysics observation well SCV. The average (R/RA) of samples from the Geophysics observation well SCV is 0.10 ± 0.003 RA, whereas the Geochemistry observation well SCV and Geochemistry observation well production casing annulus have R/RA of 0.15 ± 0.07 and 0.15 ± 0.01, respectively. The R/RA of the samples from the three wells is similar, with a greater range of R/RA ratios from the Geochemistry observation well SCV, likely due to mixing with atmospheric air. This R/RA value is close to the crustal average for the western Canadian sedimentary basin (Hiyagon and Kennedy 1992). The average 40Ar/36Ar is 311.1 ± 8.7 for samples from the Geophysics observation well SCV, which displays resolvable contributions from deeper crustal sources, 295.8 ± 0.2 for the Geochemistry observation well SCV and 295.8 ± 0.2 for the Geochemistry observation well production casing annulus, the latter two of which are within error of air. To obtain this observed isotopic composition of the sample from the Geophysics observation well SCV would require considerable time and/or significant interactions with rock formations with large amounts of 40K (Kazemi et al. 2006) and elevated temperatures (Hunt et al. 2012).
There is only a slight difference in δ13CCH4 and δ2HCH4 of gas from the Geophysics observation well SCF and Injection SCV as compared against the Geochemistry Observation well SCV and Geochemistry Observation well production casing annulus, however there is a more distinguishable argon isotopic signature between SCV gases from the two wells (no argon isotope results were available for the Injection well SCV). It should also be noted, that gas from the Geophysics observation well SCF and Injection well SCV can be distinguished by on average slightly higher helium concentrations from the Geophysics well SCV. The addition of non hydrocarbon gases allows for improved differentiation of gas composition.
Conclusion
The concentrations of N2, O2, Ar and He in gases emitted from surface casing vent flows were statistically within the same range (specifically, the average result plus/minus one standard deviation of each method overlapped with each other) when measured using a GC and a miniRUEDI mass spectrometer. Similarly, concentrations measured using a MSMS and miniRuedi were statistically within the same range for He and Ar (specifically the average plus/minus one standard deviation of each method overlapped with each other). One might intuitively expect that using laboratory-based instrumentation would generate results with lower uncertainty and higher accuracy; however, over time concentration results determined in the field were within error of those measured in the laboratory. Some variations are likely attributable to field sample handling, transportation, and pre-analytical storage of samples. In addition to comparing these different methods for measuring concentrations of noble gases, this study illustrates that non-reactive gas analysis can help with improved differentiation of subsurface gas sources, which may allow for them to be used as additional tracer.
Data availability
All data are included.
References
Alberta Energy Regulator, 2021a, Directive 20. Well abandonment.
Aslanyan A, Aslanyan I, Karantharath R, Minakhmetova R, Kohzadi H, Ghanavati M (2015) Spectral noise logging integrated with high-precision temperature logging for a multi-well leak detection survey in South Alberta. Society of petroleum engineers offshore Europe exhibition and conference, Aberdeen, UK, SPE-1754502015
Bachu S (2017) Analysis of gas leakage occurrence along wells in Alberta, Canada, from a GHG perspective – Gas migration outside well casing. Int J Greenhouse Gas Control 61:146–154. https://doi.org/10.1016/j.ijggc.2017.04.003
Boyer G (2016a) In-situ well integrity seminar/workshop, Calgary, June 30th, 2017, Alberta Energy Regulator, 23 p. (PDF last accessed July 4th, 2016a, could not be accessed subsequently).
Boyer G (2016b) Vent Flow/Gas Migration Data Trends in the Western Provinces (abstract), Optimizing Resources: Geoconvention 2016b, Calgary March 7–11, 2016b 1 p., https://geoconvention.com/wp-content/uploads/abstracts/2016b/283_GC2016b_Vent_Flow_Gas_Migration_Data_Trends_Western_Provinces.pdf (accessed July 6th, 2020).
Brantley SL, Yoxtheimer D, Arjmand S, Grieve PV, R, Pollak, R, Llewellyn, GT., Abad, J. and Simon C, (2014) Water resource impacts during unconventional shale gas development: The Pennsylvania experience. Int J Coal Geol 126:140–156. https://doi.org/10.1016/j.coal.2013.12.017
Brennwald MS, Schmidt M, Oser J, Kipfer R (2016) A Portable and Autonomous Mass Spectrometric System for On-Site Environmental Gas Analysis. Environ Sci Technol 50(24):13455–13463. https://doi.org/10.1021/acs.est.6b03669
Ballentine CJ Burgess R and Marty B (2002) Chapter 13: Tracing Fluid Origin, Transport and Interaction in the Crust, In: Noble Gases in Geochemistry and Cosmochemistry. Reviews in Mineralogy and Geochemistry Ed. Vol. 47 D. Porcelli D, Ballentine CJ and Wieler R pp 539–414.
Cahill AG, Steelman CM, Forde O, Kuloyo O, Ruff ES, Mayer B, Mayer KU, Strous M, Ryan CM, Cherry JA, Parker BL (2017) Mobility and persistence of methane in groundwater in a controlled-release field experiment. Nat Geosci 10:289–294. https://doi.org/10.1038/ngeo2919
Cahill AG, Parker BL, Mayer B, Mayer KU, Cherry JA (2018) High resolution spatial and temporal evolution of dissolved gases in groundwater during a controlled natural gas release experiment. Sci Total Environ 622–623:1178–1192. https://doi.org/10.1016/j.scitotenv.2017.12.049
Cahill AG, Beckie R, Ladd B, Sandl E, Goetz M, Chao J, Soares J, Manning C, Chopra C, Finke N, Hawthorne I, Black A, Mayer UK, Crowe S, Cary T, Lauer R, Mayer B, Allen A, Kirste D, Welch L (2019) Advancing knowledge of gas migration and fugitive gas from energy wells in northeast British Columbia. Canada Greenhouse Gases: Science and Technology 9(2):134–151. https://doi.org/10.1002/ghg.1856
Castro MC, Jambon A, de Marsily G, Schlosser P (1998) Noble gases as natural tracers of water circulation in the Paris Basin: Measurements and discussion of their origin and mechanisms of vertical transport in the basin. Water Resour Res 34(10):2443–2466. https://doi.org/10.1029/98WR01956
Cherry J, Ben-Eli M, Bharadwaj L, Chalaturnyk R, Dusseault M, Goldstein B, Lacoursière J.-P, Matthews R, Mayer, B, Molson, J, Munkittrick, K, Oreskes, N, Parker, E, Young, P, (2014). Environmental impacts of shale gas extraction in Canada: The Expert Panel on Harnessing Science and Technology to Understand the Environmental Impacts of Shale Gas Extraction, Council of Canadian Academies. Ottawa (ON): Council of Canadian Academies. 292 p. Retrieved July 6, 2020 from: http://www.scienceadvice.ca/uploads/eng/assessments%20and%20publications%20and%20news%20releases/shale%20gas/shalegas_fullreporten.pdf
Cheung K. and Mayer B (2009) Chemical and isotopic characterization of shallow groundwater from selected monitoring wells in Alberta Part 1: 2006–2007. Retrieved from: http://www.assembly.ab.ca/lao/library/egovdocs/2009/alen/173473.pdf last accessed July 6th, 2020.
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Cody JD, Hutcheon IE (1994) Regional water and gas geochemistry of the Mannville Group and associated horizons, southern Alberta. Bulletin of Canadian Petroleum Geology 42(4):449–464
Darrah TH (2018) Time to settle the fracking controversy. Groundwater 56(2):161–162. https://doi.org/10.1111/gwat.12636
Darrah TH, Poreda RJ (2012) Evaluating the accretion of meteoritic debris and interplanetary dust particles in the GPC-3 sediment core using noble gas and mineralogical tracers. Geochim Cosmochim Acta 84:329–352. https://doi.org/10.1016/j.gca.2012.01.030
Darrah TH, Vengosh A, Jackson RB, Warne N, Poreda RJ (2014) Noble gases identify the mechanisms of fugitive gas contamination in drinking-water wells overlying the Marcellus and Barnett Shales. Proc Natl Acad Sci 111(39):14076–14081. https://doi.org/10.1073/pnas.1322107111
Darrah TH, Jackson RB, Vengosh A, Warner NR, Whyte CJ, Walsh TB, Kondash AJ, Poreda RJ (2015a) The evolution of Devonian hydrocarbon gases in shallow aquifers of the northern Appalachian Basin: Insights from integrating noble gas and hydrocarbon geochemistry. Geochim Cosmochim Acta 170:321–355. https://doi.org/10.1016/j.gca.2015.09.006
Darrah TH, Jackson RB, Vengosh A, Warner NR, Poreda RJ (2015b) Noble gases: a new technique for fugitive gas investigation in groundwater. Groundwater 53(1):23–28
Dusseault MB, Jackson RE, and MacDonald D (2014) Towards a Road Map for mitigating the rates and occurrences of long-term wellbore leakage. Contract Report to the Alberta Energy Regulator. 69 p. retrieved from: http://geofirma.com/wp-content/uploads/2015/05/lwp-final-report_compressed.pdf
Dusseault M, Jackson R (2014) Seepage pathway assessment for natural gas to shallow groundwater during well stimulation, in production, and after abandonment. Environ Geosci 21(3):107–126. https://doi.org/10.1306/eg.04231414004
Forde ON, Cahill AG, Mayer KU, Mayer B, Simister RL, Finke N, Crowe SA, Cherry JA, Parker BL (2019) Hydro-biogeochemical impacts of fugitive methane on a shallow unconfined aquifer. Sci Total Environ 690:1342–1354. https://doi.org/10.1016/j.scitotenv.2019.06.322
Godwin R, Abouguendia Z, Thorpe J (1990) Lloydminster Area Operators Gas Migration Team Response of Soils and Plants to Natural Gas Migration from Two Wells in the Lloydminster Area, Saskatchewan Research Council, E-2510–3-E-90, 85 p
Hardie D and Lewis A (2015) Understanding and mitigating well integrity challenges in a mature basin. In: Presentation Made at IOGGC Meeting. September 29, Tulsa, OK, USA. http://iogcc.ok.gov/Websites/iogcc/images/2015OKC_Presentations/Understanding_and_Mitigating_Well-Integrity_Challenges-Hardie-Lewis.pdf
Harkness JS, Darrah TH, Warner NR, Whyte CJ, Moore MT, Millot R, Kloppmann W, Jackson RB, Vengosh A (2017) The geochemistry of naturally occurring methane and saline groundwater in an area of unconventional shale gas development. Geochim Cosmochim Acta 208:302–334. https://doi.org/10.1016/j.gca.2017.03.039
Heilweil VM, Solomon DK, Darrah TH, Gilmore TE, Genereux DP (2016) Gas-tracer experiment for evaluating the fate of methane in a coastal plain stream: degassing versus in-stream oxidation. Environ Sci Technol 50(19):10504–10511. https://doi.org/10.1021/acs.est.6b02224
Hendry MJ, Barbour SL, Schmeling EE, Mundle SOC, Huang M (2016) Fate and transport of dissolved methane and ethane in cretaceous shales of the Williston Basin, Canada. Water Resour Res 52(8):6440–6450. https://doi.org/10.1002/2016WR019047
Hendry MJ, Schmeling EE, Barbour SL, Huang M, Mundle SOC (2017) Fate and transport of shale-derived. Biogenic Methane Nature Scientific Reports 7(1):4881. https://doi.org/10.1038/s41598-017-05103-8
Hitchon B (1963a) Geochemical studies of natural gas, Part I hydrocarbons in western canadian natural gases. J Canadian Petrol Tech 2(2):60–76
Hitchon B (1963) Geochemical studies of natural gas, part ii acid gases in western canadian natural gases. J Canadian Petrol Tech 2(3):100–116
Hitchon B (1963c) Geochemical Studies of Natural Gas, Part III Inert gases in western Canadian Natural Gases. J Canad Petrol Tech 2(3):165–174
Hiyagon H, Kennedy M (1992) Noble gases in CH4 rich gas fields, Alberta, Canada. Geochem Cosmochem Acta 56:1569–1589. https://doi.org/10.1016/0016-7037(92)90226-9
Hudson F (2004) RSKSOP-175, Sample Preparation and Calculation for Dissolved Gas Analysis in Water Samples Using GC Headspace Equilibration Technique, EPA document, p 1–17
Humez P, Mayer B, Ing J, Nightingale M, Becker V, Kingston A, Akbilgic O, Taylor S (2016a) Occurrence and origin of methane in groundwater in Alberta (Canada): gas geochemical and isotopic approaches. Sci Total Environ 541:1253–1258. https://doi.org/10.1016/j.scitotenv.2015.09.055
Humez P, Mayer B, Nightingale M, Ing J, Becker V, Jones D, Lam V (2016b) An 8-year record of gas geochemistry and isotopic composition of methane during baseline sampling at a groundwater observation well in Alberta (Canada). Hydrogeol J 24:109–122. https://doi.org/10.1007/s10040-015-1319-1
Humez P, Mayer B, Nightingale M, Becker V, Kingston A, Taylor S, Bayegnak G, Millot R, Kloppmann W (2016c) Redox controls on methane formation, migration and fate in shallow aquifers. Hydrol Earth Syst Sci 20:1–19. https://doi.org/10.5194/hess-20-2759-2016
Hunt AG, Darrah TH, Poreda RJ (2012) Determining the source and genetic fingerprint of natural gases using noble gas geochemistry: a northern Appalachian Basin case study. AAPG Bull 96(10):1785–1811. https://doi.org/10.1306/03161211093
Hutcheon I (1999) Controls on the distribution of non-hydrocarbon gases in the Alberta Basin. Bullet Canad Petrol Geol 47(4):573–593
Jackson RB, Vengosh A, Darrah TH, Warner NR, Down A, Poreda RJ, Osborn SG, Zhao K, Karr J (2013) Increased stray gas abundance in a subset of drinking water wells near Marcellus shale gas extraction. Proc Natl Acad Sci 110(28):11250–11255. https://doi.org/10.1073/pnas.1221635110
Jackson RB, Vengosh A, Carey W, Davis RJ, Darrah TH, Francis O, Pétron G (2014) The environmental costs and benefits of fracking. Annu Rev Environ Resour 39:327–362. https://doi.org/10.1146/annurev-environ-031113-144051
Jackson RB, Pearson BR, Osborn SG, Warner NR, Vengosh A (2011) Research and policy recommendations for hydraulic fracturing and shale‐gas extraction. Center on Global Change, Duke University, Durham, NC. 12 p. retrieved from: https://nicholas.duke.edu/cgc/HydraulicFracturingWhitepaper2011.pdf, accessed July 6th, 2020
James A, Burns B (1984) Microbial alteration of subsurface natural gas accumulations. AAPG Bull 68(8):957–960
Kampbell D, Vandegrift S (1998) Analysis of dissolved methane, ethane and ethylene in ground water by a standard gas chromatographic technique. J Chromatogr Sci 36:253–256. https://doi.org/10.1093/chromsci/36.5.253
Kang M, Christian S, Celia MA, Mauzerall DL, Bill M, Miller AR, Chen Y, Conrad ME, Darrah TH, Jackson RB (2016) Identification and characterization of high methane-emitting abandoned oil and gas wells. Proc Natl Acad Sci 113(48):13636–13641. https://doi.org/10.1073/pnas.1605913113
Kazemi GA, Lehr JH, Perrochet P (2006) Chapter 6: Age-dating very old groundwaters in groundwater age. Wiley, New Jersey, pp 189–191
Kelly W, Matisoff G, Fisher JB (1985) The effects of a gas well blow out on groundwater chemistry. Environ Geol 7(4):205–213
Kreuzer RL, Darrah TH, Grove BS, Moore MT, Warner NR, Eymold WK, Whyte CJ, Mitra G, Jackson RB, Vengosh A (2018) Structural and hydrogeological controls on hydrocarbon and brine migration into drinking water aquifers in southern New York. Groundwater 56(2):225–244. https://doi.org/10.1111/gwat.12638
Mayer B, Humez P, Becker V, Nightingale M, Ing J, Kingston A, Clarkson C, Cahillb A, Parkerb E, Cherry J, Millot R, Kloppmann W, Osadetz K, Lawton D (2015) Prospects and limitations of chemical and isotopic groundwater monitoring to assess the potential environmental impacts of unconventional oil and gas development. Procedia Earth Planetary Science 13:320–323. https://doi.org/10.1016/j.proeps.2015.07.076
McIntosh JC, Hendry MJ, Ballentine C, Haszeldine RS, Mayer B, Etiope G, Elsner M, Darrah TH, Prinzhofer A, Osborn S, Stalker L, Kuloyo O, Lu ZT, Martini A, Lollar BS (2019) A critical review of state-of-the-art and emerging approaches to identify fracking-derived gases and associated contaminants in aquifers. Environ Sci Tech 53(3):1063–1077. https://doi.org/10.1021/acs.est.8b05807
Moore MT, Phillips SC, Cook AE, Darrah TH (2020) Improved sampling technique to collect natural gas from hydrate-bearing pressure cores. Appl Geochem 122:104773. https://doi.org/10.1016/j.apgeochem.2020.104773
Noomen MF, van der Werff HMA, van der Meer FD (2012) Spectral and spatial indicators of botanical changes caused by long-term hydrocarbon seepage. Eco Inform 8:55–64. https://doi.org/10.1016/j.ecoinf.2012.01.001
Standards Norway (2013) Well integrity in drilling and well operations: Standard D-010 Rev. 4, June 2013, NORSOK Standard Online AS, 224p , https://www.standard.no/petroleum (accessed June 22, 2020)
Palcsu L, Kompár L, Deák J, Szucs P, Papp L (2017) Estimation of the natural groundwater recharge using tritium-peak and tritium/helium-3 dating techniques in Hungary. Geochem J 51(5):439–448. https://doi.org/10.2343/geochemj.2.0488
Phillips NG, Ackley R, Crosson ER, Down A, Hutyra LR, Brondfield M, Karr JD, Zhao K, Jackson RB (2013) Mapping urban pipeline leaks: Methane leaks across Boston. Environ Pollut. https://doi.org/10.1016/j.envpol.2012.11.003
Alberta Energy Regulator, 2021b, Directive 87. Well integrity management.
Romanak KD, Sherk GW, Yang C, Hovorka SD (2013) Assessment of alleged CO2 leakage at the Kerr farm using a simple process-based soil gas technique: Implications for carbon capture, utilization, and storage (CCUS) monitoring. Proceedings of the 11th conference on greenhouse gas control technologies, 18–22 November 2012, Kyoto Japan, Energy Procedia 37:4242–4248
Sandau, C. D, 2016a, Characterizing the source zones for surface casing vent leaks, GeoConvention 2016a: Calgary
Sandau CD (2016b) Characterizing the source zones for surface casing vent leaks, GeoConvention 2016b: Calgary
Schoell M (1983) Genetic characterization of natural gases. AAPG Bull 67(12):2225–2238
Stolper DA, Martini AM, Clog M, Douglas PM, Shusta SS, Valentine DL, Sessions AL, Eiler JM (2015) Distinguishing and understanding thermogenic and biogenic sources of methane using multiply substituted isotopologues. Geochim Cosmochim Acta 161:219–247. https://doi.org/10.1016/j.gca.2015.04.015
Summers RJ (2010) Alberta water well survey – Report prepared for Alberta Environment, University of Alberta, 106 p. https://open.alberta.ca/dataset/a6ef970f-923c-4297-8e29-0a61707a1d9f/resource/76f9edb0-bff9-4790-b47b-ab05d33e4eab/download/albertawaterwellsurvey-report-dec2010.pdf (accessed July 6, 2020)
Szatkowski B, Whittaker S and Johnston B (2002) Identifying the source of migrating gases in surface casing vents and soils using stable carbon isotopes, Golden Lake Pool, west-central Saskatchewan, in Summary of Investigations 2002, v. 1, Saskatchewan Geological Survey, Sask. Industry and Resources Misc. Report 2002–4.1, p. 118–125
Thermo Fisher Scientific (2011) GC Isolink Operating Manual, Revision D-1222980
Tilley B, Muehlenbachs K (2012) Fingerprinting of gas contaminating groundwater and soils in a petroliferous region, Alberta, Canada; in: Environmental Forensics: Proceedings of the 2011 INEF Conference. PDF eISBN: 978–1–84973–496–7, DOI:https://doi.org/10.1039/9781849734967-00115
Tilley B, Muehlenbachs K (2006) Gas maturity and alteration systematics across the Western Canada Sedimentary Basin from four mud gas isotope depth profiles. Org Geochem 37(12):1857–1868. https://doi.org/10.1016/j.orggeochem.2006.08.010
Utting N, Clark ID, Lauriol B, Wieser M, Aeschbach-Hertig W (2012) Origin and flow dynamics of perennial groundwater in continuous permafrost terrain using isotopes and noble gases: case study of the fishing Branch River, Northern Yukon, Canada. Permafrost and Periglacial Processes 23(2):91–106. https://doi.org/10.1002/ppp.1732
Van Stempvoort D, Maathuis H, Jaworski E, Mayer B, Rich K (2005) Oxidation of fugitive methane in ground water linked to bacterial sulfate reduction. Groundwater 43(2):187–199
Vengosh A, Jackson RB, Warner N, Darrah TH, Kondash A (2014) A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ Sci Technol 48(15):8334–8348. https://doi.org/10.1021/es405118y
Vinson DS, Blair NE, Martini AM, Larter S, Orem WH, McIntosh JC (2017) Microbial methane from in situ biodegradation of coal and shale: a review and re-evaluation of hydrogen and carbon isotope signatures. Chem Geol 453(20):128–145. https://doi.org/10.1016/j.chemgeo.2017.01.027
Watson TL, Bachu S (2004) Surface casing vent flow repair – A process: PAPER 2004–297. 5th Canadian international petroleum conference, Canadian Institute of Mining, Metallurgy and Petroleum, Petroleum Society of Canada, June 8–10, 2004, Calgary, p. 8. https://www.onepetro.org/download/conference-paper/PETSOC-2004-297?id=conference-paper%2FPETSOC-2004-297: accessed June 22, 2020
Watson TL, Bachu S (2009) Evaluation of the potential for gas and CO2 leakage along wellbores. SPE Drilling Complet 24(1):115–126. https://doi.org/10.2118/106817-PA
Weiss R (1971a) The Effect of Salinity on the Solubility of Argon in Seawater: Deep Sea Research and Oceanographic Abstracts 18(2):225–230
Weiss R (1971b) Solubility of helium and neon in water and seawater. J Chem Eng Data 16(2):235–241
Wigston A, Ryan D, Osadetz K, Hubert C, Hamuli C, Watson T, Casorso D, Ewen D, McPherson R, Pavlakos P, Heseltine J, Zahacy T, Walsh R, Heagle D, Williams J (2019) Technology Roadmap to Improve Wellbore Integrity: Summary Report. Natural Resources Canada, CANMETEnergy, 85 p: https://www.nrcan.gc.ca/science-and-data/research-centres-and-labs/canmetenergy-research-centres/technology-roadmap-improve-wellbore-integrity/21964: accessed March 11, 2021
Wisen J, Chesnaux R, Werring J, Wendling J, Baudron P, Barbecot F (2020) A portrait of wellbore leakage in northeastern British Columbia, Canada. Proc Natl Acad Sci USA 117(2):913–922. https://doi.org/10.1073/pnas.1817929116
Acknowledgements
The authors would like to acknowledge financial support from the Government of Canada’s Office of Energy Research and Development (OERD).
Funding
Open Access provided by Natural Resources Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: S.Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Utting, N., Osadetz, K., Darrah, T.H. et al. Methods and benefits of measuring non-hydrocarbon gases from surface casing vents. Int. J. Environ. Sci. Technol. 20, 5223–5240 (2023). https://doi.org/10.1007/s13762-022-04300-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04300-x