Abstract
Petroleum oil contaminants have become severe ecological problems and negatively impact human health. It is, therefore, imperative to identify environmentally friendly approaches to remediate oil-polluted environments. Therefore, bacterial oil degradation stimulated with a nitrogen source under optimum conditions was assessed in this study. Based on the 16S rRNA analysis, strain ODB H32 recovered from oil-based mud of some petroleum drilling sites in the western desert, Egypt, was identified as Enterobacter hormaechei. The metabolic fingerprint of E. hormaechei, achieved using BIOLOG GEN III, revealed that the strain could utilize diverse carbon and chemical sources. Also, E. hormaechei could biodegrade 0.6% of oil under optimized pH (7.0) and temperature (30 °C) conditions. Analyzing different nitrogen stimulants revealed that peptone ˃ yeast extract ˃ ammonium nitrate ˃ urea enhanced the growth of E. hormaechei on mineral salts medium (MSM). Analysis by capillary gas chromatography revealed maximum (70.7%) degradation of peptone by E. hormaechei, indicating that peptone was a good biostimulant for oil degradation. These findings recommend using biostimulated E. hormaechei as an eco-friendly approach for remediating oil-polluted environments, under optimized conditions, especially in arid regions like the western desert of Egypt.
Similar content being viewed by others
Introduction
Crude oil is the most widespread contaminant within marine waters, rivers, lakes, sediments, and soil (Ozyurek and Bilkay, 2020; El-Liethy et al. 2017). Pollution of these environments could arise from numerous sources. For example, oil pollution in aquatic environments might be due to leakage during shipping activities and pipeline failure during petroleum transportation (Santisi et al. 2015). Also, oil contamination in the soil may result from household waste or could be due to human errors in industrial settings and pipelines explosion (FAO, 2018).
Oil-based mud is used as an anti-corrosive agent during the petroleum drilling process because of its excellent lubricating features and stability at high temperatures. However, the large amount of oil-based mud waste generated from this process is often disposed of in the environment without treatment, leading to potentially severe adverse impacts on humans, animals, and the environment (Onuh et al. 2020; Xu and Xu, 2019). In humans, oil pollutants have been associated with poor mental, physical, and physiological health and cause genetic, immune, and endocrine disruptions (Laffon et al. 2016). In aquatic ecosystems, these pollutants are linked to reproductive failures, physiological stress, and even death in aquatic life (Ji et al. 2021). In addition, drastic reductions in seed germination, soil microbial community intoxication, and reduced soil fertility have been reported where these oil pollutions have occurred on land (Malik et al. 2021). Thus, the devastating effects of environmental oil pollution necessitate effective remediation methods.
Methods for remediating oil-contaminated aquatic environments can be physical (mechanical), chemical, and biological (EPA, 2017). Mechanical methods may include stationary floating devices to control the oil spill (Gapingsi et al. 2017) and in situ burning (Graham et al. 2016). Chemical treatment methods may involve spraying dispersants on the oil-polluted environment, breaking the pollutant into minute droplets (Kleindienst et al. 2015). The lighter oil portions are evaporated or removed photo-oxidatively and geochemically, while heavier portions are dissolved; some fractions can be removed through biodegradation by naturally occurring microflora, especially bacteria (Wang et al. 2021).
Biodegradation is an eco-friendly approach in which organic compounds are degraded into simple compounds by microorganisms, i.e., bacteria, fungi, or protozoa (Kachieng’A and Momba, 2017; Yaman, 2020). These microorganisms utilize organic pollutants as a carbon source. Bacteria that can utilize oil are called oil-degrading bacteria (ODB) (Varjani and Upasani et al. 2021).
There are two oil biodegradation approaches. The first approach is bioaugmentation, in which exogenous live microorganisms are added to a polluted site to break down harmful oil pollutants into non-toxic components like carbon dioxide and water (Varjani and Upasani et al. 2021; Tzirita, 2012). However, this approach is limited by the high mortality of the inoculated microorganisms and their slow dispersal in a solid matrix. The high mortality could be due to (1) biotic factors like competition with indigenous microorganisms for nutrients and predation by protozoa, and (2) abiotic factors such as pH, temperature, and moisture to which the organisms need to adapt (Garbisu et al. 2017; Nzila et al. 2016; Quan et al. 2010). The second approach is biostimulation, whereby the bioremediation ability of indigenous microorganisms is enhanced by adding nutrients (Li et al. 2021; Chibuike et al. 2014). Despite the overall success of bioremediation approaches, the microorganisms' biodegradation potentials can be affected by parameters like temperature, pH, aeration, and phosphorus and nitrogen sources availability (Liu et al. 2017). Also, fluctuations in pH, temperature, and salt concentration in the environment cause oil pollutants to form aggregative colloidal particles that precipitate, slowing the microorganisms' activities (Boglaienko and Tansel, 2018). With these challenges, it is imperative to identify suitable organisms that can biodegrade oil and determine optimal conditions to enhance their activities in oil-polluted milieus. Therefore, the present study assessed the ability of a novel Enterobacter hormaechei strain ODB H32, isolated from petroleum oil-based muds (OBM), for oil biodegradation following stimulation with nitrogen sources and determined the optimum conditions for the process.
Materials and methods
Study site and sample collection
Eleven grab oil-based muds (OBM) waste samples were obtained from various petroleum sites in Abu Sennan, El Alamein, Qattara Depression, Burg El Arab, Marina, and El Hamra in the western desert, Egypt (Fig. 1). The samples were sealed in sterile plastic ziplock bags and transported to the laboratory in an icebox for analysis (APHA/AWWA/WEF, 2017).
Evaluation of the total bacterial counts
The total bacterial count (TBC) was performed at two incubation temperatures (22 and 30 °C) to determine the natural mesophilic bacterial flora in the oil-based mud samples during the study. These temperatures represented the average daily temperatures during sampling. The TBC was determined through the pour plate method, following APHA’s guidelines (APHA/AWWA/WEF, 2017). Briefly, samples (10 g) were transferred into 90 mL sterile distilled water. One mL of the serially diluted sample was innoculated on plate count agar (Difco™) and incubated at 30 ± 0.5 °C for 24 h. A second set of plates supplemented with 50 µg/mL nystatin to inhibit fungal growth was incubated at 22 ± 0.5 °C for 48 h. An uninoculated plate served as a control. After the incubation, the bacterial counts were expressed as colony-forming unit (CFU)/g according to the following formula:
Isolation and purification of oil-degrading bacteria (ODB)
Oil-degrading bacteria were enumerated by spread plating on oil agar (OA) medium, according to Ijah and Antai (2003), supplemented with 1 ppm of oil purchased from Cooperative Company for Petroleum (COOP), Egypt. After incubating at 30 °C for five days, distinct colonies were randomly selected and subcultured twice on fresh OA plates to obtain pure cultures before storage at -20 °C in tryptic soy broth (TSB) (BD, Germany).
Screening of oil-degrading bacterial isolates
Forty-five pure isolates were selected from all positive plates, and their oil utilization potential was screened as previously described (Okpokwasili and Okorie, 1988). Briefly, 100 μL from a fresh TSB culture of the isolates was inoculated into 10 mL of sterile Bushnell Haas Broth (BHB; Sigma-Aldrich, AGITECH, Cairo) (Xia et al. 2006) and 0.1% (V/V) oil. A reaction mixture without bacteria served as a control. The isolates were screened at different oil concentrations, 0.2, 0.3, 0.4, 0.5, and 0.6% (V/V), according to Das and Mukherjee (2007). The ODB growth was assessed by streaking samples from the BHB tubes onto OA and plate count agar and incubating at 30 °C for five days and 24 h, respectively.
Molecular identification of isolates
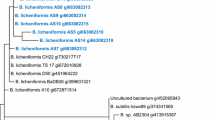
Genomic DNA was extracted from the most potent oil-degrading isolate after resuscitation in TSB using the Presto™ Mini gDNA bacterial kit (Geneaid, Taiwan) according to the manufacturer’s instructions. The amplified and purified 16S rRNA gene for each isolate was sent to Macrogen (Soul, South Korea) for sequencing on a 3730XL DNA Analyzer (Applied BioSystems, USA). Sequence similarity search was performed through the NCBI BLAST online tool against the nucleotide collection (nr/nt) database. The identified Enterobacter hormaechei strain has been deposited at NCBI GeneBank under accession number MK691687.1. In addition, a phylogenetic tree for Enterobacter hormaechei was constructed using MEGA 7.0 (Kumar et al. 2016).
Metabolic fingerprint of Enterobacter hormaechei
The metabolic fingerprint of E. hormaechei for 71 carbon sources and 23 chemical sensitivity tests was ascertained using BOLOG GEN III (El-Liethy et al. 2018). After incubating for 18–24 h, results were automatically captured through the computerized MicroStation™ system (BiologInc., USA) with software preloaded fingerprint data (OmniLog® Data Collection).
Determination of optimum conditions for the biodegradation
Different pH, temperature, and nitrogen sources were tested to determine optimum biodegradation process conditions.
Preparation of inoculum
E. hormaechei was inoculated to 100 mL of TSB (BD, Germany). The flask was incubated at 30 °C for 18–24 h. Following incubation, bacteria were pelleted by centrifugation (4000 rpm, 15 min) in a Sigma 2-16P centrifuge, washed twice with 0.87% NaCl saline at neutral pH, and resuspended in normal saline, obtaining an optical density of 0.6- 1.0 (OD600).
Determination of the optimum temperature and pH and the most suitable nitrogen sources
The optimum temperature for biodegradation was determined by exposing the E. hormaechei strain to different temperatures, including 20, 25, 30, 35, and 40 °C. For the pH, values of 6.0, 7.0, 7.5, 8.0, and 8.5 were tested after adjustment with either 1 N of HCl or 1 N of NaOH. For the most suitable nitrogen source, ammonium nitrate (NH4NO3) was used as an inorganic nitrogen source, while peptone, yeast extract, and urea were used as organic nitrogen sources. Each nitrogen source was tested individually in BHB at a concentration of 0.6% (w/v) and ambient temperature of 30 °C and pH 7. In each experiment, a 1-L flask containing 500 mL of BHB was inoculated with 0.6% crude oil (v/v) and 1 mL of E. hormaechei suspension with an initial count of 106 CFU/mL. Each flask was sampled after 0, 1, 2, 3, 5, 7, 10, 14, 21, and 30 days, and the biodegradation was assessed by measuring the bacterial cell density as CFU/mL.
E. hormaechei oil biodegradation under optimized conditions
Biodegradation of oil using E. hormaechei was carried out at fixed optimized conditions of 30 °C temperature and pH 7.0. The prepared E. hormaechei strain (106 CFU/mL) was inoculated into 500 mL of sterile BHB with 0.6% oil (v/v) as the only carbon source. Two nitrogen sources, ammonium nitrate, and peptone were used separately as stimulants. The control consisted of an uninoculated flask. The flasks were incubated at 30 °C for 30 days, and the biodegradation rate was determined using capillary gas chromatography (CGC).
Capillary gas chromatography (CGC)
Chloroform was used to extract the remaining oil from the BHB following ASTM standard protocols (ASTM-D2007-19; https://www.astm.org/Standards/D2007.htm) and analyzed through gas chromatography as described in ASTM-D6730-01 (https://www.astm.org/Standards/D6730.htm).
Statistical analysis
The relationship between TBC at 22 and 30 °C and ODB (R2) was investigated using linear regression. Also, the correlations between the measured parameters were ascertained using the Pearson correlation with GraphPad Prism 5.0. Analyses were performed at an alpha = 0.05.
Results and discussion
Determination of total and oil-degrading bacterial counts
The total bacterial count (TBC) in oil-contaminated mud samples was measured at two incubation temperatures (22 and 30 °C). The average TBC at 22 °C and 30 °C was 1.4 × 104 and 1.6 × 104 CFU/g, respectively. The least TBC was observed at the Burg El Arab site (1.2 × 103 and 5.0 × 102 CFU/g at 22 and 30 °C), while the highest TBC was observed at the El Hamra site (8.0 × 105 and 6.0 × 104 CFU/g at 22 and 30 °C). Figure 2 shows that the sampled oil-based mud recorded comparatively higher TBC at 22 °C than at 30 °C.
The ODB counts ranged between 1.0 × 102 and 6.1 × 103 CFU/g (Fig. 2), with the highest ODB counts observed at the Abu Sennan site, followed by Qattara Depression and El Hamra.
Statistically, the TBC at 22 °C was significantly and positively correlated to ODB counts (p < 0.05). Moreover, a significantly positive correlation was observed between TBC at 22 °C and 30 °C (p < 0.05) and between the TBC at 30 °C and ODB counts (p < 0.05) (Fig. 1). In this study, the percentage of TBC represented by the ODB at 30 °C was between 3.0 and 90%, with Abu Sennan and El Hamra containing the highest percentages of ODB, followed by the Qattara Depression site.
The bacterial isolate growing well at 0.6 (V/V) oil concentration was identified by sequencing the 16S rRNA gene and was closely related to E. hormaechei ODBH32 (Fig. 3). This sequence is deposited in Genbank (accession number MK691687.1). The metabolic fingerprinting revealed that the organism could grow in a broad pH and sodium chloride range and diverse sugar sources (Table S1; Supplementary Materials).
A phylogenetic tree was constructed with the 16S rRNA sequence of the most potent oil-degrading strain E. hormaechei ODBH32 (marked in red) based on a maximum likelihood analysis. The accession number of E. hormaechei ODBH32 strain is MK691687.1. E. hormaechei strain EB_P9_L5 (ST90) was used as the reference type strain
Factors affecting the biodegradation rate
Effect of temperature
The E. hormaechei counts and activities increased with increasing temperatures from 20, 25, 30, and 35 °C for thirty days. Specifically, there was only a slight increase (0.28–1.72 logs) in the density of E. hormaechei at 20, 25, and 35 °C. However, at 30 °C, there was a significant increase of 2.46 logs during the study period (Fig. 4). Therefore, the optimum temperature for the oil biodegradation was observed at 30 °C, followed by 25 °C and 35 °C. Figure 4 illustrates that at 40 °C, the cell density started declining on day 14 of the experiment. Thus, the order of the optimum temperature was 30 °C ˃ 25 °C ˃ 35 °C ˃20 °C ˃40 °C.
Effect of pH
E. hormaechei cell density slightly increased by one log at pH values 6.0 and 7.5. However, the optimum growth was observed at pH 7.0, whereas the cell density increased by 1.16 log after day 7 and 2.54 logs after day 30. On the other hand, the cell density decreased slightly by 1.9 logs after day 7.0 at pH 8.5 and by 2.2 logs after day 21 (Fig. 5). The order of the most optimum pH was 7.0 ˃ 7.5 & 6.0 ˃ 8.0 ˃ 8.5.
Effect of nitrogen source
The present study revealed that peptone was the most suitable nitrogen source favoring bacterial biodegradation, followed by yeast extract, then NH4NO3 (Fig. 6), as adding peptone increased E. hormaechei’s growth rate by 2.61 log10 (Fig. 6). On the other hand, the lowest increase in the growth of E. hormaechei (1.27 log10) was observed with the addition of urea (Fig. 6). Thus, the order of the optimum nitrogen source was peptone ˃ yeast extract ˃ ammonium nitrate ˃ urea.
Determination of oil biodegradation using Capillary Gas Chromatography (CGC)
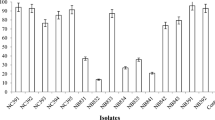
Analysis by CGC revealed a variable oil degradation ability of E. hormaechei both in the presence of ammonium nitrate and peptone (Fig. 7). Isolate C37 completely degraded oil in the presence of ammonium nitrate, while isolates C9, 291 C12, C24, and C37 of E. hormaechei demonstrated complete degradation in the presence of peptone (Fig. 7). While comparing the impact of two substrates, the biodegradation rate was maximum with peptone compared to those recorded for ammonium nitrate.
Discussion
Determination of total and oil-degrading bacterial counts
The isolation of microorganisms from oil-contaminated environments and their reintroduction into the same environment remains more effective in oil degradation than using exogenous organisms from different environments (Aburto-Medina et al. 2012). In the current study, an indigenous E. hormaechei strain that could degrade oil under optimized biodegradation conditions was isolated from oil-based mud. Also, the high TBC recorded confirms that an increase in nutrient load due to pollution could increase the total environmental microbial population (Zhang et al. 2020). Furthermore, bacteria bind to mud surfaces, thereby accumulating in oil-based mud (Xia et al. 2006). This could further explain the highest TBC (around 104 CFU/g) recorded in the current study. However, the average TBC at 22 °C and 30 °C recorded in the current study was slightly lower than those observed in a study carried out by Imarhiagbe and Atuanya (2014), in which the authors reported mean TBC ranging between 5.4 × 105 and 7.23 × 105 CFU/g. The observed difference could be because of slight differences in the analyzed samples. In their study, they analyzed water-based and oil-based mud, while the current study only focused on oil-based mud.
It has been shown that there is a correlation between the presence of ODB and the degradation of oil pollutants in the environment (Sadeghi Haddad Zavareh et al. 2016; El-Liethy et al. 2017). The highest ODB count in the current study was observed at Abu Sennan, one of the largest oil fields in Egypt that has received extensive oil pollution over the years. This field covers an area of over 3600 km2 (Abuseda et al. 2016). This could, therefore, account for the high ODB counts observed at this site compared to the other sites.
There was a positive correlation between TBC and ODB. These findings corroborate those of Balogun et al. (2014), who reported that TBC and ODB in auto-mechanic samples ranged from 1.0 × 106 to 2.8 × 106 CFU/g and 4.0 × 105 to 2.0 × 106 CFU/g, respectively. Previous studies had attributed the high TBC:ODB ratios in an active Ekofisk oil field in Norway to the occurrence of oil pollutants in the sediment of that region (Oppenheimer et al. 1977; Atlas, 1981). Similarly, Obafemi et al. (2018) found that the TBC ranged between 2.6 × 107 and 4.5 × 107 CFU/g in oil-contaminated soil, while the ODB count was between 1.2 and 1.5 × 104 CFU/g; they observed that the percentage of ODB in the TBC was less than 0.5%.
In the present study, after six screening steps for ODB, it was found that only one bacterial isolate out of a total of 45 isolates could grow at 0.6 (v/v) oil concentrations. This could be because fast bacterial growth occurred at low oil concentrations, while the growth rate became slower and suppressed at higher oil concentrations. One mechanism involved in extremely high oil concentration is the alteration of the bacterial cell surface, leading to cell death (Hou et al. 2018). Furthermore, such alterations may damage the membrane, impacting membrane functions, inhibiting cell growth, and releasing cell content due to cell lysis (Galitskaya et al. 2021). Additionally, extremely high oil concentrations have been proved to disrupt the nutrient cycle, thereby reducing microbial growth (Xu et al. 2018). Similarly, the higher oil concentration could reduce the overall microbial biomass (Labud et al. 2007).
Sequencing the 16S rRNA gene revealed the isolated bacterium as E. hormaechei strain ODBH32. This organism has been previously isolated from oil-contaminated environments (Khanafer et al. 2017; Mojarad et al. 2016). The metabolic fingerprint examination revealed that E. hormaechei could grow in a wide range of NaCl and pH and utilize numerous chemical and sugar substrates. Thus, genotypic identification of microorganisms followed by metabolic fingerprint gives a better understanding of their environmental distribution and microbial behavior (Lucas and Manna, 2013; Sandle et al. 2013; El-Liethy et al. 2018).
Factors affecting the biodegradation rate
Numerous physical, chemical, and biological factors, influence oil biodegradation (Arjoon and Speight, 2020). Previous biodegradation studies revealed that pH ranges between 7.0 and 7.5 favored some bacteria’s oil biodegradation abilities (El-Liethy et al. 2017; Samhan et al. 2017). On the other hand, it has also been shown that sufficient concentrations of nutrients and oxygen could improve bacteria's optimal growth rate for oil degradation at a pH between 6 and 9 (Das and Chandran 2011). Also, temperature directly alters the chemical structure of oil pollutants and the physiology and diversity of the degrading microorganisms (Margesin and Schinner, 2001; Venosa and Zhu, 2003).
Effect of temperature
In the current study, the bacterial cell density increased at 30 °C than the other tested temperatures, indicating that 30 °C was the optimum temperature for the oil biodegradation. Previous studies have reported mesophilic temperatures (~ 20 to 35 °C) as more suitable and efficient for biodegradation than very low or high temperatures (Boopathy, 2000; Naeem and Qazi, 2020). Similarly, some studies have reported optimum temperatures of between 30 °C and 37 °C to activate aerobic bacterial enzymes for hydrocarbon biodegradation (Hamzah et al. 2011; Vaidya et al. 2017). Temperature changes also indirectly impact ODB by modifying the oil pollutant. At low temperatures, the oil's viscosity increases and consequently, the solubility and volatilization decrease. Such conditions prolong the presence of toxic organic compounds in the environment and delay the biodegradation commencement (Ribicic et al. 2018). Also, the biodegradation temperature could be affected by the type of environment in which the process occurs. For instance, some studies reported the highest degradation rates between 30 and 40 °C in soil, while similar outputs were achieved in the aquatic milieus between 15 and 30 °C (Bartha and Bossert, 1984).
Effect of pH
Optimizing pH is crucial for improving bacterial growth and enhancing the biodegradation of oils (Palanisamy et al. 2014). In the present study, pH 7 was considered the optimum pH for oil biodegradation. The effect of pH is attributed to bacteria's enzymatic activities being pH-dependent (Yuniati, 2018). For example, a previous study found that E. hormaechei optimally biodegraded oil at pH 7.0 (Sivagamasundari and Jayakumar, 2017). On the other hand, deviations to a more acidic and alkaline pH did not support the bacterial growth (Margesin and Schinner, 2001; Vaidya et al. 2018). In the current study, fluctuations in cell density were observed at pH 7.5 (Fig. 5). E. hormaechei cell density increased in the first week and then declined until the second week before increasing to 30 days. A pH of 7.5 is close to neutrality; hence, the fluctuations could have been due to bacterial acclimatization to the new environment. This could also explain why the cell density at pH 6 was like that at pH 7.5 after 30 days. Similarly, Bamforth and Singleton (2005) observed that the biodegradation percentage increased twofold from 40% at acidic pH to 80% at a neutral pH.
Effect of nitrogen source
Adding nutrients like carbon, nitrogen, and phosphorus enhances oil biodegradation (Jin and Fallgren, 2007). Nitrogen plays an essential role in microbial cell synthesis. However, this could vary from one nitrogen source to another. Consequently, adding a nitrogen source is required to encourage pollutant biodegradation (Hesnawi and Adbeib, 2013). The current study found that peptone gave the maximum E. hormaechei growth rate as an organic nitrogen source, favoring maximum bacterial biodegradation. Peptone has been identified as the best nitrogen source compared to urea, yeast extract, and inorganic nitrogen for oil biodegradation (Hamzah et al. 2012; Ibrahim et al. 2013, 2005; Sivagamasundari and Jayakumar, 2017). Yeast extract is an essential nutrient required by many bacterial strains to produce enzymes. In this study, the lowest increase in the growth rate of E. hormaechei was obtained with urea. Although the study conducted by Jin and Fallgren (2007) reported that urea preferentially enhanced biodegradation due to its high nitrogen content, other studies have indicated contradictory findings. For instance, it has been shown that urea negatively impacts oil biodegradation due to its high ammonia content and acid toxicity, which significantly reduces the microbial population (Sarkar et al. 2016). On the other hand, the addition of ammonium nitrate had minimal effect on the microbial biomass production, hence the biodegradation rate, because of nitrate ions that inhibit certain microorganisms (Hamzah et al. 2012).
Determination of oil biodegradation using Capillary Gas Chromatography (CGC)
Microbes' metabolic activities are affected by nutrient deficiency, lowering the biodegradation rate. Biostimulation means supplementing microbes with nutrients to improve biodegradation (Azubuike et al. 2016). Lately, the biodegradation of aromatic hydrocarbons has been enhanced by biostimulating the bacterial strains involved (Li et al. 2021). In the present study, the oil biodegradation rate was measured using CGC after 30 days at pH 7.0 and 30 °C. The removal efficiency using E. hormaechei, supplemented with peptone, was higher (70.7%) than with ammonium nitrate (51.63%). About 50% of light oil (C9-C20) and heavy oil (C22 and C24) was biodegraded using ammonium nitrate. Total biodegradation for C9, C12, C24, and C37 was observed by stimulating with peptone. This means that peptone is a good stimulant during oil biodegradation (Mukred et al. 2008). In recent studies, oil biodegradation has been enhanced by biostimulation of microorganisms after 63 (Yamen, 2020), six (Li et al. 2021), and 56 (Varjani and Upasani et al. 2021) days incubation.
Conclusion
We isolated Enterobacter hormaechei ODB H32 (accession number MK691687.1) from oil-based mud collected from petroleum sites in Egypt's western desert and screened it for its oil biodegradation potentials. This isolate's metabolic fingerprint determined using BIOLOG GEN III revealed that E. hormaechei could grow in 0.6% (v/v) oil as a carbon and energy source. Furthermore, the optimum growth rate of E. hormaechei for oil biodegradation in BHB medium was achieved at pH 7.0 and a temperature of 30 °C. Also, the biodegradation percentage was higher with peptone (70.7%) as a stimulant than ammonium nitrate (51.63%). These findings recommend using biostimulated E. hormaechei as an environmentally friendly approach for remediating oil-polluted environments, under optimized conditions, especially in arid regions like the western desert of Egypt.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary materials.
References
Aburto-Medina A, Adetutu EM, Aleer S, Weber J, Patil SS, Sheppard PJ, Ball AS, Juhasz AL (2012) Comparison of indigenous and exogenous microbial populations during slurry phase biodegradation of long-term hydrocarbon-contaminated soil. Biodegradation 23:813–822
APHA/AWWA/WEF (2017) Standard Methods for the Examination of Water and Wastewater, 23rd ed., American Public Health Association, Washington, DC. ISBN 9780875532356
Arjoon K, Speight JG (2020) Chemical and physical analysis of a petroleum hydrocarbon contamination on a soil sample to determine its natural degradation feasibility. Inventions 5:43
Bamforth SM, Singleton I (2005) Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol 80:723–736. https://doi.org/10.1002/jctb.1276
Boopathy R (2000) Factors limiting bioremediation technologies. Bioresour Technol 74:63–67. https://doi.org/10.1016/S0960-8524(99)00144-3
Chibuike GU, Obiora SC, Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:1–12. https://doi.org/10.1155/2014/752708
Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011:1–13. https://doi.org/10.4061/2011/941810
Das K, Mukherjee AK (2007) Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour Technol 98:1339–1345. https://doi.org/10.1016/j.biortech.2006.05.032
El-Liethy MA, Hemdan BA, Samhan FA, Ali SS, El-Taweel GE (2017) Optimizing conditions for crude oil degrading bacterial consortium isolated from aquatic environment. Pollut Res 36:197–204
El-Liethy MA, Hemdan BA, El-Taweel GE (2018) Phenotyping using semi-automated BIOLOG and conventional PCR for identification of Bacillus isolated from biofilm of sink drainage pipes. Acta Ecol Sin 38:334–338. https://doi.org/10.1016/j.chnaes.2018.01.011
EPA (2017) Oil Spill Response Techniques [WWW Document]. EPA’s Response Tech. URL https://www.epa.gov/emergency-response/epas-response-techniques (accessed 11.9.20).
Galitskaya P, Biktasheva L, Blagodatsky S, Selivanovskaya S (2021) Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci Rep 11:1–18. https://doi.org/10.1038/s41598-020-80631-4
Gapingsi GE, Korbas R, Santos M (2017) Modelling and control of a flexible floating boom: first approach. IFAC-PapersOnLine 50:13108–13113. https://doi.org/10.1016/j.ifacol.2017.08.2163
Hamzah A, Tavakoli A, Rabu A (2011) Detection of toluene degradation in bacteria isolated from oil contaminated soils. Sains Malaysiana 40:1231–1235
Hamzah A, Zarin MA, Hamid AA, Omar O, Senafi S (2012) Optimal physical and nutrient parameters for growth of Trichoderma virens UKMP-1M for heavy crude oil degradation. Sains Malaysiana 41:71–79
Hesnawi RM, Adbeib MM (2013) Effect of nutrient source on indigenous biodegradation of diesel fuel contaminated soil. APCBEE Proc 5:557–561. https://doi.org/10.1016/j.apcbee.2013.05.093
Hou N, Zhang N, Jia T, Sun Y, Dai Y, Wang Q, Li D, Luo Z, Li C (2018) Biodegradation of phenanthrene by biodemulsifier-producing strain Achromobacter sp. LH-1 and the study on its metabolisms and fermentation kinetics. Ecotoxicol Environ Saf 163:205–214. https://doi.org/10.1016/j.ecoenv.2018.07.064
Ibrahim HM, Yusoff WMW, Hamid AA, Illias RM, Hassan O, Omar O (2005) Optimization of medium for the production of β-cyclodextrin glucanotransferase using Central Composite Design (CCD). Process Biochem 40:753–758. https://doi.org/10.1016/j.procbio.2004.01.042
Ibrahim ASS, Al-Salamah AA, El-Tayeb MA, El-Badawi YB (2013) Enhancement of Amphibacillus sp NPST-10 cyclodextrin glucanotransferase production by optimizing physio-environmental factors. J Pure Appl Microbiol 7:2597–2606
Ijah UJJ, Antai SP (2003) Removal of Nigerian light crude oil in soil over a 12-month period. Int Biodeterior Biodegrad 51:93–99. https://doi.org/10.1016/S0964-8305(01)00131-7
Imarhiagbe E, Atuanya E (2014) A study of the microbiology and polycyclic aromatic hydrocarbons (PAHs) compositional profile and sources in drill cuttings from Ologbo oilfield wells at Edo State. Nigeria Sci World J 9:8–13
Jin S, Fallgren PH (2007) Site-specific limitations of using urea as a nitrogen source in biodegradation of petroleum wastes in soil. Soil Sediment Contam 16:497–505. https://doi.org/10.1080/15320380701490200
Kachieng’A L, Momba MNB (2017) Biodegradation of petroleum hydrocarbon functional groups in contaminated water using selected protozoan isolates. J Environ Chem Eng 5:1885–1891. https://doi.org/10.1016/j.jece.2017.03.022
Khanafer M, Al-Awadhi H, Radwan S (2017) Coliform bacteria for bioremediation of waste hydrocarbons. Biomed Res Int. https://doi.org/10.1155/2017/1838072
Kleindienst S, Paul JH, Joye SB (2015) Using dispersants after oil spills: Impacts on the composition and activity of microbial communities. Nat Rev Microbiol 13:388–396. https://doi.org/10.1038/nrmicro3452
Labud V, Garcia C, Hernandez T (2007) Effect of hydrocarbon pollution on the microbial properties of a sandy and a clay soil. Chemosphere 66:1863–1871. https://doi.org/10.1016/j.chemosphere.2006.08.021
Laffon B, Pásaro E, Valdiglesias V (2016) Effects of exposure to oil spills on human health: updated review. J Toxicol Environ Heal B Crit Rev 19:105–128. https://doi.org/10.1080/10937404.2016.1168730
Liu J, Bacosa HP, Liu Z (2017) Potential environmental factors affecting oil-degrading bacterial populations in deep and surface waters of the Northern Gulf of Mexico. Front Microbiol 7:1–14. https://doi.org/10.3389/fmicb.2016.02131
Margesin R, Schinner F (2001) Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl Microbiol Biotechnol 56:650–663. https://doi.org/10.1007/s002530100701
Mojarad M, Alemzadeh A, Ghoreishi G, Javaheri M (2016) Kerosene biodegradation ability and characterization of bacteria isolated from oil-polluted soil and water. J Environ Chem Eng 4:4323–4329. https://doi.org/10.1016/j.jece.2016.09.035
Mukred AM, Hamid AA, Hamzah A, Wan Yusoff WM (2008) Enhancement of biodegradation of crude petroleum-oil in contaminated water by the addition of nitrogen sources. Pak J Biol Sci 11:2122–2127. https://doi.org/10.3923/pjbs.2008.2122.2127
Okpokwasili GC, Okorie BB (1988) Biodeterioration potentials of microorganisms isolated from car engine lubricating oil. Tribol Int 21:215–220. https://doi.org/10.1016/0301-679X(88)90020-5
Quan X, Tang H, Xiong W, Yang Z (2010) Bioaugmentation of aerobic sludge granules with a plasmid donor strain for enhanced degradation of 2,4-dichlorophenoxyacetic acid. J Hazard Mater 179:1136–1142. https://doi.org/10.1016/j.jhazmat.2010.04.002
Ribicic D, McFarlin KM, Netzer R, Brakstad OG, Winkler A, Throne-Holst M, Størseth TR (2018) Oil type and temperature dependent biodegradation dynamics: combining chemical and microbial community data through multivariate analysis. BMC Microbiol 18:1–15. https://doi.org/10.1186/s12866-018-1221-9
Sadeghi Haddad Zavareh M, Ebrahimipour G, Shahriari Moghadam M, Fakhari J, Abdoli T (2016) Bioremediation of crude oil using bacterium from the coastal sediments of Kish Island. Iran Iran J Public Health 45:670–679
Santisi S, Cappello S, Catalfamo M, Mancini G, Hassanshahian M, Genovese L, Giuliano L, Yakimov MM (2015) Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium. Brazilian J Microbiol 46:377–387. https://doi.org/10.1590/S1517-838246120131276
Sarkar J, Kazy SK, Gupta A, Dutta A, Mohapatra B, Roy A, Bera P, Mitra A, Sar P (2016) Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front Microbiol 7:1–20. https://doi.org/10.3389/fmicb.2016.01407
Sivagamasundari T, Jayakumar N (2017) Optimization of diesel oil degrading bacterial strains at various culture parameters. Int J Res Dev Pharm Life Sci 6:2840–2844. https://doi.org/10.21276/ijrdpl.2278-0238.2017.6(6).2840-2844
Tzirita M (2012) A characterisation of bioaugmentation products for the treatment of waste fats, oils and grease (FOG). Dublin City University.
Vaidya S, Devpura N, Jain K, Madamwar D (2018) Degradation of chrysene by enriched bacterial consortium. Front Microbiol 9:1–14. https://doi.org/10.3389/fmicb.2018.01333
Vaidya S, Jain K, Madamwar D (2017) Metabolism of pyrene through phthalic acid pathway by enriched bacterial consortium composed of Pseudomonas, Burkholderia, and Rhodococcus (PBR). 3 Biotech 7:1–15. Doi: https://doi.org/10.1007/s13205-017-0598-8
Venosa AD, Zhu X (2003) Biodegradation of crude oil contaminating marine shorelines and freshwater wetlands. Spill Sci Technol Bull 8:163–178. https://doi.org/10.1016/S1353-2561(03)00019-7
Xia XH, Yu H, Yang ZF, Huang GH (2006) Biodegradation of polycyclic aromatic hydrocarbons in the natural waters of the Yellow River: effects of high sediment content on biodegradation. Chemosphere 65:457–466. https://doi.org/10.1016/j.chemosphere.2006.01.075
Xu P, Xu M (2019) Damage mechanism of oil-based drilling fluid flow in seepage channels for fractured tight sandstone gas reservoirs. Geofluids. https://doi.org/10.1155/2019/2672695
Xu X, Liu W, Tian S, Wang W, Qi Q, Jiang P, Gao X, Li F, Li H, Yu H (2018) Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis. Front Microbiol 9:1–11. https://doi.org/10.3389/fmicb.2018.02885
Yaman C (2020) Performance and kinetics of bioaugmentation, biostimulation, and natural attenuation processes for bioremediation of crude oil-contaminated soils. Processes 8(8):883. https://doi.org/10.3390/pr8080883
Yuniati MD (2018) Bioremediation of petroleum-contaminated soil: a review. IOP Conf Ser Earth Environ Sci 118:0–7
Acknowledgements
The authors are grateful to the Consulting Unit for Virus Research and Bioassays, National Research Centre, Egypt, for providing the facilities to conduct this research.
Funding
Open access funding provided by The Science, Technology &. No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
MAE, MME, MGE, AIH, and GEE conceptualized the work. MAE and MME carried out the laboratory analyses. MAE, MME, GEE, MGE, AIH, and ALKA interpreted the results and performed data analysis. MAE wrote the initial draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Editorial responsibility: Josef Trögl.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Liethy, M.A., El-Noubi, M.M., Abia, A.L.K. et al. Eco-friendly bioremediation approach for crude oil-polluted soils using a novel and biostimulated Enterobacter hormaechei ODB H32 strain. Int. J. Environ. Sci. Technol. 19, 10577–10588 (2022). https://doi.org/10.1007/s13762-021-03885-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03885-z