Abstract
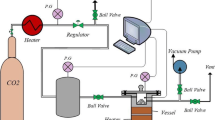
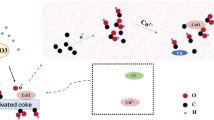
In this research, activated carbon (AC)-based absorbents modified with NiO and MgO were prepared by wet impregnation method for adsorption of carbon dioxide (CO2). The effect of adding (Ni(NO3)2 6(H2O)) and (Mg(NO3)2 6(H2O)) in 1, 3, 5, and 7 wt% to AC was studied. Raw AC and modified AC were characterized by ultimate analysis, scanning electron microscopy, X-ray diffraction, and surface area. In addition, response surface methodology method was used to optimize the adsorption operation condition. The five-level central composite design was applied to design the experiments for three types of adsorbents (AC, AC/NiO-3, and AC/MgO-3) in the temperature and pressure ranges of 25–80 °C and 2–10 bar, respectively. The results indicated that the adsorption capacity of activated carbon was modified after NiO and MgO loading, especially at higher temperatures, and the optimal concentrations were obtained 3 wt% for both of them. For better evaluation of the adsorbents behavior, experimental data were investigated by isotherm, kinetic, and thermodynamic models. The optimum adsorption capacities were obtained 121.35, 105.17 mg/g for AC/NiO-3 and AC/MgO-3, respectively.

















Similar content being viewed by others
References
Adamski A et al (2007) Surface modification of ZrO2 nanopowder with oxovanadium species using slurry deposition and impregnation methods. J Alloy Compd 442(1–2):302–305
Adelodun AA et al (2016) Isotherm, thermodynamic and kinetic studies of selective CO2 adsorption on chemically modified carbon surfaces. Aerosol Air Qual Res 16:3312–3329
Aharoni C, Tompkins FC (1970) Kinetics of adsorption and desorption and the Elovich equation. Adv Catal 21(C):1–49
Ahmed MJ, Dhedan SK (2012) Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilib 317:9–14
Amiri M, Shahhosseini S, Ghaemi A (2017) Optimization of CO2 capture process from simulated flue gas by dry regenerable alkali metal carbonate based adsorbent using response surface methodology. Energy Fuels 31(5):5286–5296
Bahadori A, Vuthaluru HB (2009) New method accurately predicts carbon dioxide equilibrium adsorption isotherms. Int J Greenh Gas Control 3(6):768–772
Brockner W, Ehrhardt C, Gjikaj M (2007) Thermal decomposition of nickel nitrate hexahydrate, Ni (NO3)2·6H2O, in comparison to Co (NO3)2·6H2O and Ca (NO3)2 4H2O. Thermochim Acta 456(1):64–68
Carabineiro SA, McKee DW, Silva IF (2001) Uncatalysed and catalysed CO2 reaction using metal catalysts and binary vanadium mixtures supported on activated carbon. Carbon 39(3):451–463
Das B et al (2013) Removal of copper from aqueous solution using alluvial soil of Indian origin: equilibrium, kinetic and thermodynamic study. J Mater Environ Sci 4(4):392–408
Deraz NM (2018) The comparative jurisprudence of catalysts preparation methods: I. Precipitation and impregnation methods. J Ind Environ Chem 2(1):19–21
Fashi F, Ghaemi A, Moradi P (2019) Piperazine-modified activated alumina as a novel promising candidate for CO2 capture: experimental and modeling. Greenh Gases Sci Technol 9(1):37–51
Fenrong Li et al (2010) Adsorption of carbon dioxide by coconut activated carbon modified with Cu/Ce. J Rare Earths 28:334–337
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57(1):385–470
Gardner TJ, Messing GL (1984) Magnesium salt decomposition and morphological development during evaporative decomposition of solutions. Thermochim Acta 78(1–3):17–27
Ghaedi AM et al (2019) Optimization and modeling of simultaneous ultrasound-assisted adsorption of ternary dyes using copper oxide nanoparticles immobilized on activated carbon using response surface methodology and artificial neural network. Ultrason Sonochem 51:264–280
Hakim A et al (2015) Study of CO2 adsorption and desorption on activated carbon supported iron oxide by temperature programmed desorption. Jurnal Teknologi 77(33):75–84
Hameed BH, Salman JM, Ahmad AL (2009) Adsorption isotherm and kinetic modeling of 2, 4-D pesticide on activated carbon derived from date stones. J Hazard Mater 163(1):121–126
Henning K-D, Schäfer S (1993) Impregnated activated carbon for environmental protection. Gas Sep Purif 7(4):235–240
Herawan SG et al (2013) Characterization of activated carbons from oil-palm shell by CO2 activation with no holding carbonization temperature. Sci World J. https://doi.org/10.1155/2013/624865
Heydarifard M et al (2018) Reactive absorption of CO2 into piperazine aqueous solution in a stirrer bubble column: modeling and experimental. Int J Greenhouse Gas Control 79:91–116
Hidayu AR, Muda N (2016) Preparation and characterization of impregnated activated carbon from palm kernel shell and coconut shell for CO2 capture. Procedia Eng 148:106–113
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Hosseini S et al (2015) Adsorption of carbon dioxide using activated carbon impregnated with Cu promoted by zinc. J Taiwan Inst Chem Eng 52:109–117
Houshmand A, Daud WMAW, Shafeeyan MS (2011) Exploring potential methods for anchoring amine groups on the surface of activated carbon for CO2 adsorption. Sep Sci Technol 46(7):1098–1112
Jang D-I, Park S-J (2012a) Influence of nickel oxide on carbon dioxide adsorption behaviors of activated carbons. Fuel 102:439–444
Khajeh Amiri M, Ghaemi A, Arjomandi H (2019) Experimental, kinetics and isotherm modeling of carbon dioxide adsorption with 13X zeolite in a fixed bed column. Iranian J Chem Eng (IJChE) 16(1):54–64
Khajeh M, Ahad G (2019) Nanoclay montmorillonite as an adsorbent for CO2 capture: experimental and modeling. J Chinese Chem Soc. https://doi.org/10.1002/jccs.201900150
Kim B-J, Cho K-S, Park S-J (2010) Copper oxide-decorated porous carbons for carbon dioxide adsorption behaviors. J Colloid Interface Sci 342(2):575–578
Lagregren S (1898) About the theory of so-called adsorption of soluble substances. Kungl Sven Veten Akad Handl 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part L. Solids. J Am Chem Soc 38(11):2221–2295
Madura JD , Herring FG, Petrucci RH, Bissonnette C (1972) General chemistry: principles and modern applications
Madzaki H et al (2018) Carbon dioxide adsorption on activated carbon hydrothermally treated and impregnated with metal oxides. J Kejuruter 30(1):31–38
Míguez JL et al (2018) Evolution of CO2 capture technology between 2007 and 2017 through the study of patent activity. Appl Energy 211:1282–1296
Mikuli E et al (2001) Melting and thermal decomposition of [Ni (H2O)6](NO3)2. Thermochim Acta 370(1–2):65–71
Mohammad NK, Ghaemi A, Tahvildari K (2019) Hydroxide modified activated alumina as an adsorbent for CO2 adsorption: experimental and modeling. Int J Greenhouse Gas Control 88:24–37
Karbalaei Mohammad N et al (2019) Experimental investigation and modeling of CO2 adsorption using modified activated carbon. Iran J Chem Chem Eng (IJCCE) 39(1):177–192
Norouzbahari S, Shahhosseini S, Ghaemi A (2016) Chemical absorption of CO2 into an aqueous piperazine (PZ) solution: development and validation of a rigorous dynamic rate-based model. RSC Adv 6(46):40017–40032
Pashaei H et al (2020) Experimental Modeling and Optimization of CO2 Absorption into Piperazine Solutions Using RSM-CCD Methodology. ACS Omega. https://doi.org/10.1021/acsomega.9b03363
Pietrzak R, Morawski AW (2013) MgO/CaO-loaded activated carbon for carbon dioxide capture: practical aspects of use. Ind Eng Chem Res
Plaza MG et al (2010) Ammoxidation of carbon materials for CO2 capture. Appl Surf Sci 256(22):6843–6849
Rashidi NA, Yusup S (2016) An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J CO2 Util 13:1–16
Saeidi M et al (2018) Exploiting response surface methodology (RSM) as a novel approach for the optimization of carbon dioxide adsorption by dry sodium hydroxide. J Chin Chem Soc 65(12):1465–1475
Saeidi M, Ghaemi A, Tahvildari K (2019) CO2 capture exploration on potassium hydroxide employing response surface methodology, isotherm and kinetic models. Iran J Chem Chem Eng (IJCCE) 39(5):255–267
Sarrai AE et al (2016) Using central composite experimental design to optimize the degradation of tylosin from aqueous solution by photo-fenton reaction. Materials 9(6):428
Schwickardi M et al (2002) High-surface-area oxides obtained by an activated carbon route. Chem Mater 14(9):3913–3919
Shafeeyan MS et al (2011) Ammonia modification of activated carbon to enhance carbon dioxide adsorption: effect of pre-oxidation. Appl Surf Sci 257(9):3936–3942
Shafeeyan MS et al (2012) The application of response surface methodology to optimize the amination of activated carbon for the preparation of carbon dioxide adsorbents. Fuel 94:465–472
Shekhawat D, Luebke DR, and Pennline HW (2003) A review of carbon dioxide selective membranes: a topical report. National Energy Technology Laboratory (NETL), Pittsburgh, PA, Morgantown, WV
Sips R (1948) On the structure of a catalyst surface. J Chem Phys 16(5):490–495
Siriwardane RV et al (2001) Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 15(2):279–284
Somy A et al (2009) Adsorption of carbon dioxide using impregnated activated carbon promoted by Zinc. Int J Greenhouse Gas Control 3(3):249–254
Son S-J et al (2005) Development of carbon dioxide adsorbents using carbon materials prepared from coconut shell. Korean J Chem Eng 22(2):291–297
Taheri FS et al (2019) High CO2 adsorption on amine-functionalized improved mesoporous silica nanotube as an eco-friendly nanocomposite. Energy Fuels 33(6):5384–5397
Thommes M et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069
Tuinier MJ et al (2010) Cryogenic CO2 capture using dynamically operated packed beds. Chem Eng Sci 65(1):114–119
Versteeg GF, Van Swaaij WPM (1988) Solubility and diffusivity of acid gases (carbon dioxide, nitrous oxide) in aqueous alkanolamine solutions. J Chem Eng Data 33(1):29–34
Wang M et al (2011) Post-combustion CO2 capture with chemical absorption: a state-of-the-art review. Chem Eng Res Des 89(9):1609–1624
Yi H et al (2014) Simultaneous removal of SO 2, NO, and CO2 on metal-modified coconut shell activated carbon. Water Air Soil Pollut 225(5):1965
Yong Z, Mata VG, Rodrigues AE (2001) Adsorption of carbon dioxide on chemically modified high surface area carbon-based adsorbents at high temperature. Adsorption 7(1):41–50
Younas M et al (2016) Feasibility of CO 2 adsorption by solid adsorbents: a review on low-temperature systems. Int J Environ Sci Technol 13(7):1839–1860
Zhou K, Li L, Ma X, Mo Y, Chen R, Li H, Li H (2018) Activated carbons modified by magnesium oxide as highly efficient sorbents for acetone. RSC Adv 8(6):2922–2932
Zieliński M et al (2005) Hydrogen storage on nickel catalysts supported on amorphous activated carbon. Catal Commun 6(12):777–783
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility Samareh Mirkia.
Rights and permissions
About this article
Cite this article
Ghaemi, A., Mashhadimoslem, H. & Zohourian Izadpanah, P. NiO and MgO/activated carbon as an efficient CO2 adsorbent: characterization, modeling, and optimization. Int. J. Environ. Sci. Technol. 19, 727–746 (2022). https://doi.org/10.1007/s13762-021-03582-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03582-x