Abstract
Background
Pivotal studies have reported a significant proportion of patients achieving no evidence of disease activity (NEDA) after 2 cycles of treatment with alemtuzumab (ATZ), that can be maintained for several years. Long-term real-world evidence regarding ATZ as well as subsequent treatment trajectories is still scarce.
Objective
To analyze the effectiveness and safety of ATZ-treated patients in a tertiary care Belgian center.
Methods
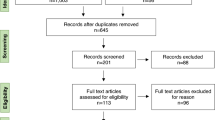
A retrospective cohort study including 32 patients treated with ATZ between 2015 and 2021 was performed.
Results
32 patients received 2 ATZ courses with a mean follow-up (FU) duration of 5.6 years (range: 2.25–8.2). 21.75% patients were treatment naïve. 40.5% were previously treated with natalizumab or fingolimod. NEDA-3 was achieved in 61.3–85% of patients, with failure mostly attributed to recurrence of radiological disease activity. During FU, annualized relapse rates remained very low (0.06–0.14), disability improvement occurred in up to 40.5%, whereas disability worsening occurred in up to 13.5%. Retreatment risk was associated with younger age (< 45 years old, Odds Ratio 8.0, p = 0.02) and a higher number of previous DMTs (Hazard ratio 2.7, 95%CI 1.3–7.4, p = 0.02). Safety in our cohort was consistent with the known profile of ATZ. At the end of FU, 65.6% patients remained untreated after 2 or 3 courses of ATZ, while the remaining switched to anti-CD20 therapy or cladribine.
Conclusion
ATZ is a high efficacy therapy for active MS, providing long-term remission in a significant proportion of patients. Retreatment was more frequent in younger patients or patients having failed a higher number of previous DMTs.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Data are located in controlled access data storage at the Cliniques Universitaires Saint-Luc.
References
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378(2):169–180. https://doi.org/10.1056/NEJMra1401483
Lublin FD, Haring DA, Ganjgahi H, Ocampo A, Hatami F, Cuklina J, Aarden P, Dahlke F, Arnold DL, Wiendl H, Chitnis T, Nichols TE, Kieseier BC, Bermel RA (2022) How patients with multiple sclerosis acquire disability. Brain 145(9):3147–3161. https://doi.org/10.1093/brain/awac016
Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, Fitzgerald KC, Song A, Liu P, Lin JP, Wu T, Johnson KR, McGavern DB, Schafer DP, Calabresi PA, Reich DS (2021) A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597(7878):709–714. https://doi.org/10.1038/s41586-021-03892-7
He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, Sharmin S, Horakova D, KubalaHavrdova E, Spelman T, Izquierdo G, Eichau S, Trojano M, Lugaresi A, Hupperts R, Sola P, Ferraro D, Lycke J, Grand’Maison F, Prat A, Girard M, Duquette P, Larochelle C, Svenningsson A, Petersen T, Grammond P, Granella F, Van Pesch V, Bergamaschi R, McGuigan C, Coles A, Hillert J, Piehl F, Butzkueven H, Kalincik T, Group MSS (2020) Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 19(4):307–316. https://doi.org/10.1016/S1474-4422(20)30067-3
Brown JWL, Coles A, Horakova D, Havrdova E, Izquierdo G, Prat A, Girard M, Duquette P, Trojano M, Lugaresi A, Bergamaschi R, Grammond P, Alroughani R, Hupperts R, McCombe P, Van Pesch V, Sola P, Ferraro D, Grand’Maison F, Terzi M, Lechner-Scott J, Flechter S, Slee M, Shaygannejad V, Pucci E, Granella F, Jokubaitis V, Willis M, Rice C, Scolding N, Wilkins A, Pearson OR, Ziemssen T, Hutchinson M, Harding K, Jones J, McGuigan C, Butzkueven H, Kalincik T, Robertson N, Group MSS (2019) Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 321(2):175–187. https://doi.org/10.1001/jama.2018.20588
Wiendl H, Carraro M, Comi G, Izquierdo G, Kim HJ, Sharrack B, Tornatore C, Daizadeh N, Chung L, Jacobs AK, Hogan RJ, Wychowski LV, Van Wijmeersch B, Care-Ms IC-M, II, Investigators C, (2020) Lymphocyte pharmacodynamics are not associated with autoimmunity or efficacy after alemtuzumab. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000000635
European public assessment report for Lemtrada (2020). www.emaeuropaeu/en/medicines/human/EPAR/lemtrada Accessed 6 August 2023
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA, investigators C-MI, (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828. https://doi.org/10.1016/S0140-6736(12)61769-3
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Miller T, Fisher E, Sandbrink R, Lake SL, Margolin DH, Oyuela P, Panzara MA, Compston DA, investigators C-MI, (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380(9856):1829–1839. https://doi.org/10.1016/S0140-6736(12)61768-1
Ziemssen T, Thomas K (2017) Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 10(10):343–359. https://doi.org/10.1177/1756285617722706
Coles AJ, Arnold DL, Bass AD, Boster AL, Compston DAS, Fernandez O, Havrdova EK, Nakamura K, Traboulsee A, Ziemssen T, Jacobs A, Margolin DH, Huang X, Daizadeh N, Chirieac MC, Selmaj KW (2021) Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord 14:1756286420982134. https://doi.org/10.1177/1756286420982134
Prosperini L, Annovazzi P, Boffa L, Buscarinu MC, Gallo A, Matta M, Moiola L, Musu L, Perini P, Avolio C, Barcella V, Bianco A, Farina D, Ferraro E, Pontecorvo S, Granella F, Grimaldi LME, Laroni A, Lus G, Patti F, Pucci E, Pasca M, Sarchielli P, Italian Alemtuzumab Study G (2018) No evidence of disease activity (NEDA-3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36-month real-world study. J Neurol 265(12):2851–2860. https://doi.org/10.1007/s00415-018-9070-x
Theodorsdottir A, Debrabant B, Magyari M, Kant M, Rasmussen PV, Malmberg CF, Norberg IA, Hansen V, Bech D, Schmidt MF, Schreiber K, Frederiksen JL, Sellebjerg F, Illes Z (2021) Alemtuzumab treatment in Denmark: a national study based on the danish multiple sclerosis registry. Mult Scler 27(14):2254–2266. https://doi.org/10.1177/13524585211003291
Ziemssen T, Bass AD, Berkovich R, Comi G, Eichau S, Hobart J, Hunter SF, LaGanke C, Limmroth V, Pelletier D, Pozzilli C, Schippling S, Sousa L, Traboulsee A, Uitdehaag BMJ, Van Wijmeersch B, Choudhry Z, Daizadeh N, Singer BA, Care-Ms IC-MIC, investigators T, (2020) Efficacy and safety of alemtuzumab through 9 years of follow-up in patients with highly active disease: post hoc analysis of care-ms i and ii patients in the TOPAZ extension study. CNS Drugs 34(9):973–988. https://doi.org/10.1007/s40263-020-00749-x
Frau J, Coghe G, Lorefice L, Fenu G, Musu L, Cocco E (2019) Efficacy and safety of alemtuzumab in a real-life cohort of patients with multiple sclerosis. J Neurol 266(6):1405–1411. https://doi.org/10.1007/s00415-019-09272-6
Russo CV, Sacca F, Frau J, Annovazzi P, Signoriello E, Bonavita S, Grasso R, Clerico M, Cordioli C, Laroni A, Capobianco M, Torri Clerici V, Sartori A, Cavalla P, Maniscalco GT, La Gioia S, Caleri F, Giugno A, Iodice R, Carotenuto A, Cocco E, Fenu G, Zaffaroni M, Baroncini D, Lus G, Gallo A, De Mercanti SF, Lapucci C, Di Francescantonio V, Brambilla L, Sormani MP, Signori A (2022) A real-world study of alemtuzumab in a cohort of Italian patients. Eur J Neurol 29(1):257–266. https://doi.org/10.1111/ene.15121
Moiola LDC M, Zanetta C, Brambilla L, Rinaldi F, Annovazzi P, Frau J, Lus G, Malucchi S, Puorro G, Bianco MA, Marfia G, Cavalla P, Cerqua R, Gallo A, Lapucci C, Filippi M, ALEMNaive study group (2022) Poster 714. A long term multicenter observational study on the efficacy and safety of alemtuzumab in multiple sclerosis- highly active naïve patients. Mult Scler J 28:130–691
Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung HP, Havrdova E, Schippling S, Selmaj KW, Traboulsee A, Compston DAS, Margolin DH, Thangavelu K, Chirieac MC, Jody D, Xenopoulos P, Hogan RJ, Panzara MA, Arnold DL, Care MS II, Investigators C (2017) Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 89(11):1117–1126. https://doi.org/10.1212/WNL.0000000000004354
Havrdova E, Arnold DL, Cohen JA, Hartung HP, Fox EJ, Giovannoni G, Schippling S, Selmaj KW, Traboulsee A, Compston DAS, Margolin DH, Thangavelu K, Rodriguez CE, Jody D, Hogan RJ, Xenopoulos P, Panzara MA, Coles AJ, Care-Ms I, Investigators C (2017) Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 89(11):1107–1116. https://doi.org/10.1212/WNL.0000000000004313
Hunter SF, Aburashed RA, Alroughani R, Chan A, Dive D, Eichau S, Kantor D, Kim HJ, Lycke J, Macdonell RAL, Pozzilli C, Scott T, Sharrack B, Wiendl H, Chung L, Daizadeh N, Baker DP, Vermersch P, Care-Ms IC-MIC, Investigators T (2021) Confirmed 6-month disability improvement and worsening correlate with long-term disability outcomes in alemtuzumab-treated patients with multiple sclerosis: post hoc analysis of the CARE-MS studies. Neurol Ther 10(2):803–818. https://doi.org/10.1007/s40120-021-00262-3
Chirikov V, Ma I, Joshi N, Patel D, Smith A, Giambrone C, Cornelio N, Hashemi L (2019) Cost-effectiveness of alemtuzumab in the treatment of relapsing forms of multiple sclerosis in the United States. Value Health 22(2):168–176. https://doi.org/10.1016/j.jval.2018.08.011
Walter E, Berger T, Bajer-Kornek B, Deisenhammer F (2019) Cost-utility analysis of alemtuzumab in comparison with interferon beta, fingolimod, and natalizumab treatment for relapsing-remitting multiple sclerosis in Austria. J Med Econ 22(3):226–237. https://doi.org/10.1080/13696998.2018.1556668
Rauma I, Mustonen T, Seppa JM, Ukkonen M, Mannikko M, Verkkoniemi-Ahola A, Kartau M, Saarinen JT, Luostarinen L, Simula S, Ryytty M, Ahmasalo R, Sipila JOT, Pieninkeroinen I, Tapiola T, Remes AM, Kuusisto H (2022) Safety of alemtuzumab in a nationwide cohort of Finnish multiple sclerosis patients. J Neurol 269(2):824–835. https://doi.org/10.1007/s00415-021-10664-w
Dayan CM, Lecumberri B, Muller I, Ganesananthan S, Hunter SF, Selmaj KW, Hartung HP, Havrdova EK, LaGanke CC, Ziemssen T, Van Wijmeersch B, Meuth SG, Margolin DH, Poole EM, Baker DP, Senior PA (2023) Endocrine and multiple sclerosis outcomes in patients with autoimmune thyroid events in the alemtuzumab CARE-MS studies. Mult Scler J Exp Transl Clin 9(1):20552173221142740. https://doi.org/10.1177/20552173221142741
Mazzitelli M, Barone S, Greco G, Serapide F, Valentino P, Giancotti A, Costa C, Pisani V, Quirino A, Liberto MC, Matera G, Gambardella A, Trecarichi EM, Torti C (2020) Listeria infection after treatment with alemtuzumab: a case report and literature review would antibiotic prophylaxis be considered? Infez Med 28(2):258–262
Van Coile L, Verhaeghe E, Ongenae K, Destrooper L, Mohamadi Z, Brochez L, Hoorens I (2023) The therapeutic dilemma of basal cell carcinoma in older adults: a review of the current literature. J Geriatr Oncol 14(3):101475. https://doi.org/10.1016/j.jgo.2023.101475
van Seijen M, Lips EH, Thompson AM, Nik-Zainal S, Futreal A, Hwang ES, Verschuur E, Lane J, Jonkers J, Rea DW, Wesseling J, team P, (2019) Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer 121(4):285–292. https://doi.org/10.1038/s41416-019-0478-6
Funding
The authors did not receive funding for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Andreea Florea and Vincent van Pesch. The first draft of the manuscript was written by Andreea Florea and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
Prof. van Pesch received travel grants from Merck Healthcare KGaA (Darmstadt, Germany), Biogen, Sanofi, Bristol Meyer Squibb, Almirall and Roche. His institution has received research grants and consultancy fees from Roche, Biogen, Sanofi, Merck Healthcare KGaA (Darmstadt, Germany), Bristol Meyer Squibb, Janssen, Almirall, Novartis Pharma, and Alexion. Prof. El Sankari and Dr. Florea have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Pesch, V., Hanganu, AR. & Sankari, S.E. Long-term follow up of alemtuzumab-treated patients: a retrospective study in a Belgian tertiary care center. Acta Neurol Belg (2024). https://doi.org/10.1007/s13760-024-02542-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13760-024-02542-9