Abstract
We surveyed 15 bodies of water among remnants of the Atlantic Forest biome in southern Brazil for adult dragonflies and damselflies to test whether an empirical selection method for diversity indicators could be applied in a subtropical ecosystem, where limited ecological knowledge on species level is available. We found a regional species pool of 34 species distributed in a nested subset pattern with a mean of 11.2 species per locality. There was a pronounced difference in species composition between spring, summer, and autumn, but no differences in species numbers between seasons. Two species, Homeoura chelifera (Selys) and Ischnura capreolus (Hagen), were the strongest candidates for regional diversity indicators, being found only at species-rich localities in our surveyed area and likewise in an undisturbed national forest reserve, serving as a reference site for the Atlantic Forest. Using our selection method, we found it possible to obtain a tentative list of diversity indicators without having detailed ecological information of each species, providing a reference site is available for comparison. The method thus allows for indicator species to be selected in blanco from taxonomic groups that are little known. We hence argue that Odonata can already be incorporated in ongoing assessment programs in the Neotropics, which would also increase the ecological knowledge of the group and allow extrapolation to other taxa.



Similar content being viewed by others
References
Almeida-Neto M, Ulrich W (2011) A straightforward computational approach for measuring nestedness using quantitative matrices. Environ Model Softw 26:173–178
Alves-Martins F, Del-Claro K, Jacobucci GB (2012) Sexual size dimorphism, mating system and seasonality of a Neotropical damselfly, Telebasis carmesina (Coenagrionidae). Int J Odonatol 15:263–273
Austin MP (2002) Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol Model 157:101–118
Beccaloni GW, Garton KJ (1995) Predicting the species richness of Neotropical forest butterflies—Ithomiinae (Lepidoptera, Nymphalidae) as indicators. Biol Conserv 71:77–86
Buss DF, Borges EL (2008) Application of Rapid Bioassessment Protocols (RBP) for benthic macroinvertebrates in Brazil: Comparison between sampling techniques and mesh sizes. Neotrop Entomol 37:288–295
Campos FS, Trindade-Filho J, Brito D, Llorente GA, Solé M (2014) The efficiency of indicator groups for the conservation of amphibians in the Brazilian Atlantic Forest. Ecol Evol 4:2505–2514
Carignan V, Villart MA (2002) Selecting indicator species to monitor ecological integrity: a review. Environ Monit Assess 78:45–61
Carle FL (1979) Environmental monitoring potential of the Odonata, with a list of rare and endangered Anisoptera of Virginia, USA. Odonatologica 8:319–323
Clausnitzer V (2003) Dragonfly communities in coastal habitats of Kenya: indication of biotope quality and the need of conservation measures. Biodiv Conserv 12:333–356
Conrad KF, Willson KH, Harvey IF, Thomas CJ, Sherratt TN (1999) Dispersal characteristics of seven odonate species in an agricultural landscape. Ecography 22:524–531
Corbet PS (1999) Dragonflies: behavior and ecology of Odonata. Cornell University Press, Ithaca, p 829
Corbet PS, Suhling F, Soendgerath D (2006) Voltinism of Odonata: a review. Intern J Odonatol 9:1–44
Cunto GC, Bernard E (2012) Neotropical bats as indicators of environmental disturbance: what is the emerging message? Acta Chiropterologica 14:143–151
D’Amico F, Darblade S, Avignon S, Blanc-Manel S, Ormerod SJ (2004) Odonates as indicators of shallow lake restoration by liming: comparing adult and larval responses. Restor Ecol 12:439–446
Dalzochio MS, Souza LOI, Uchoa MA, Costa JM (2011) First records of Odonata (Insecta) from the Bodoquena Mountains, Mato Grosso do Sul, Brazil. Entomobrasilis 4:135–138
De Marco P, Latini AO, Reis AP (1999) Environmental determination of dragonfly assemblage in aquaculture ponds. Aquac Res 30:357–364
De Marco P, Nogueira DS, Correa C, Vieira TB, Silva KD, Pinto NS, Bichsel D, Hirota ASV, Vieira RRS, Carneiro FM, Oliveira AAB, Carvalho P, Bastos RP, Ilg C, Oertli B (2014) Patterns in the organization of Cerrado pond biodiversity in Brazilian pasture landscapes. Hydrobiologia 723:87–101
INPE - Instituto Nacional de Pesquisas Espaciais (2012) Centro de Previsão de Tempo e Estudos Climáticos, Banco de Dados Meteorológicos. Ministério da Ciência e Tecnologia. http://bancodedados.cptec.inpe.br/ Accessed 4 Mar 2014
Ducatti A, Périco E, Arend U, Cemin G, Haetinger C, Rempel C (2011) Análise da paisagem por Sistemas de Informação Geográfica (SIGs) e métricas de paisagem como subsídio para a tomada de decisões em nível ambiental. Espacios 32:36–42
Favretto MA, Santos EB, Geuster CJ (2013) Entomofauna do Oeste do Estado de Santa Catarina, Sul do Brasil. Entomobrasilis 6:42–46
Fenoglio S, Badino G, Bona F (2002) Benthic macroinvertebrate communities as indicators of river environment quality: an experience in Nicaragua. Rev Biol Trop 50:1125–1131
Flenner I, Sahlén G (2008) Dragonfly community re-organisation in boreal forest lakes: rapid species turnover driven by climate change? Insect Conserv Divers 1:169–179
Garrison RW, von Ellenrieder N, Louton JA (2006) Dragonfly genera of the new world: an illustrated and annotated key to the Zygoptera. The John Hopkins University Press, Baltimore, p 384
Garrison RW, von Ellenrieder N, Louton JA (2010) Damselfly genera of the new world: an illustrated and annotated key to the Zygoptera. The John Hopkins University Press, Baltimore, p 528
Geist J (2011) Integrative freshwater ecology and biodiversity conservation. Ecol Ind 11:1507–1516
Graça MAS, Ferreira WR, Firmiano K, França J, Callisto M (2015) Macroinvertebrate identity, differed across patches differing in substrate particle size and leaf litter pack in low order, tropical Atlantic forest streams. Limnetica 34:29–40
Halme P, Mönkkönnen M, Kotiaho JS, Ylisirniö A, Markkanen A (2009) Quantifying the indicator power of an indicator species. Conserv Biol 23:1008–1016
Hannon ER, Hafernik JE (2007) Reintroduction of the rare damselfly Ischnura gemina (Odonata: Coenagrionidae) into an urban California park. J Insect Conserv 11:141–149
Harabis F, Dolný A (2010) Ecological factors determining the density-distribution of Central European dragonflies (Odonata). Eur J Entomol 107:571–577
Heckman CW (2006) Encyclopedia of South American aquatic insects: Odonata–Anisoptera. Springer, Dordrecht, p 725
Heckman CW (2010) Encyclopedia of South American aquatic insects: Odonata–Zygoptera. Springer, Washington DC, p 691
Kerr JT, Sugar A, Packer P (2000) Indicator taxa, rapid biodiversity assessment and nestedness in an endangered ecosystem. Conserv Biol 14:1726–1734
Kessler M, Bach K (1999) Using indicator families for vegetation classification in species-rich Neotropical forests. Phytocoenologia 29:485–502
Koch K, Wagner C, Sahlén G (2014) Farmland versus forest: comparing changes in Odonata species composition in western and eastern Sweden. Insect Conserv Divers 7:22–31
Leal CG, Câmara IG (2003) The Atlantic forest of South America: biodiversity status, threats and outlook. Island Press, Washington DC, p 488
Lehikoinen A (2013) Climate change, phenology and species detectability in a monitoring scheme. Pop Ecol 55:315–323
Lencioni FAA (2006) The damselflies of Brazil: an illustrated identification guide II—Coenagrionidae family. All Print Editora, São Paulo, p 419
Lewis OT (2006) Climate changes, species-area curves and the extinction crisis. Phil Trans R Soc B 361:163–171
Machado ABM (2001) Studies on neotropical protoneuridae (Odonata, Zygoptera). Rev Bras Zool 21:333–336
May RM (1998) How many species are there on Earth? Science 241:1441–1449
Monteiro CS, Couceiro SRM, Hamada N, Juen L (2013) Effect of vegetation removal for road building on richness and composition of Odonata communities in Amazonia, Brazil. Int J Odonatol 16:135–144
Nessimian JL, Venticinque EM, Zuanon J, De Marco JP, Gordo M, Fidelis L, Dar’c Batista J, Juen L (2008) Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian streams. Hydrobiologia 614:117–131
Nobrega CC, De Marco P (2011) Unprotecting the rare species: a niche-based gap analysis for odonates in a core Cerrado area. Divers Distrib 17:491–505
Norling U (1984) Photoperiodic control of larval development in Leucorrhinia dubia (Vander Linden): A comparison between populations of Southern and northern Sweden (Anisoptera: Libellulidae). Odonatologica 13:529–550
Oertli B (2008) The use of dragonflies in the assessing and monitoring of aquatic habitats. In: Córdoba-Aguilar A (ed) Model organisms for ecological and evolutionary research. Oxford University Press, Oxford, pp 79–95
Patterson BD, Atmar W (1995) Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol J Linnean Soc 28:65–82
Pérez L, Lorenschat J, Massaferro J, Pailles C, Sylvestre F, Hollwedel W, Brandorff GO, Brenner M, Islebe G, del Socorro Lozano M, Scharf B, Schwalb A (2013) Bioindicators of climate and trophic state in lowland and highland aquatic ecosystems of the Northern Neotropics. Rev Biol Trop 61:603–644
Pinto NS, Juen L, Cabette HSR, De Marco P (2012) Fluctuating asymmetry and wing size of Argia tinctipennis Selys (Zygoptera: Coenagrionidae) in relation to riparian forest preservation status. Neotrop Entomol 41:2–10
Pires MM, Kotzian CB, Spies MR, Neri DB (2013) Biota Neotropica 13:259–267
Pompeu PS, Alves CBM, Callisto M, Brown LR, Gray RH, Hughes RM (2003) The effects of urbanization on biodiversity and water quality in the Rio das Velhas basin, Brazil. Effects of Urbanization on Stream Ecosystems. Am Fish Soc Symp 47:11–22
Rapport DJ (1990) Challenges in the detection and diagnosis of pathological change in aquatic ecosystems. J Great Lakes Res 16:609–618
Ribeiro MC, Martensen AC, Metzger JP, Tabarelli M, Scarano F, Fortin M-J (2011) The Brazilian Atlantic Forest: a shrinking biodiversity hotspot. In: Zachos FE, Habel JC (eds) Biodiversity hotpots. Springer, Berlin, pp 405–434
Sætersdal M, Gjerde I, Blom HH (2005) Indicator species and the problem of spatial inconsistency in nestedness patterns. Biol Conserv 122:305–316
Sahlén G (1999) The impact of forestry on dragonfly diversity in Central Sweden. Int J Odonatol 2:177–186
Sahlén G, Ekestubbe K (2001) Identification of dragonflies (Odonata) as indicators of general species richness in boreal forest lakes. Biodivers Conserv 10:673–690
Samways MJ, Steytler NS (1995) Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management. Biol Conserv 78:279–288
Samways MJ, Caldwel PM, Osborn R (1996) Spatial patterns of dragonflies (Odonata) as indicators for design of a conservation pond. Odonatologica 25:157–166
Scotland RW, Wortley AH (2003) How many species of seed plants are there? Taxon 52:101–104
Sebastião H, Grelle CEV (2009) Taxon surrogates among Amazonian mammals: Can total species richness be predicted by single orders? Ecol Ind 9:160–166
Sick H (1982) Ornitologia Brasileira. Uma Introdução. Editora UnB, Brasília, p 862
Simaika JP, Samways MJ (2009) An easy-to-use index of ecological integrity for prioritizing freshwater sites and for assessing habitat quality. Biodiv Conserv 18:1171–1185
Simberloff D (1998) Flagships, umbrellas, and keystones: is single-species management passé in the landscape era? Biol Conserv 83:247–257
Smith E, van Belle G (1984) Nonparametric estimation of species richness. Biometrics 40:119–129
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarityof species and its application to analyses of the vegetation on Danish commons. Det Kongelige Danske Videnskabernes Selskab. Biologiske Skrifter 5:1–34
Stewart DAB, Samways MJ (1998) Conserving dragonfly (Odonata) assemblages relative to river dynamics in an African savanna game reserve. Conserv Biol 12:683–692
Suhling F, Sahlén G, Martens A, Marais E, Schütte C (2006) Dragonfly assemblage composition and diversity in arid tropical environments: a case study from western Namibia. Biodiv Conserv 15:311–332
Suhling F, Samways MJ, Simaika JP, Richter O, Marais E, Martens A, Kipping J (2010) Dragonfly diversity from the Cape to the Kavango. In: Schmiedel UN (ed) Biodiversity in southern Africa, 2: patterns and processes at regional scale. Klaus Hess Publishers, Göttingen, pp 64–69
von Ellenrieder N (2002) A phylogenetic analysis of the extant Aeshnidae (Odonata: Anisoptera). Systematic Entomol 27:437–467
von Ellenrieder N (2008) Drepaponeura gen. nov, for Epipleoneura letitia and Protoneura peruviensis, with description of eight new Protoneuridae form South America (Odonata: Protoneuridae). Zootaxa 1842:1–34
von Ellenrieder N, Molineri C, Emmerich D (2009) Odonata de Uruguay: lista de especies y nuevos registros. Ver Soc Entomol Argent 68:227–237
Wittwer T, Sahlén G, Suhling F (2010) Does one community shape the other? Dragonflies and fish in Swedish lakes. Insect Conserv Div 3:124–133
Acknowledgments
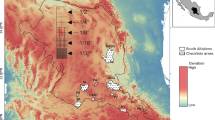
We are grateful to Daniel Martins dos Santos for helping us produce Figs 1 and 2. We would like to thank our field assistants who gave commendable assistance in our sampling efforts and also the landowners who generously gave permission for our team to use their properties during the development and implementation of this study. The project was made possible through a Linnaeus-Palme teacher and student exchange program (The International Programme Office for Education and Training, Stockholm, Sweden) between Halmstad University and Univates. Financial support was provided by FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited Kleber Del Claro – UFU
Rights and permissions
About this article
Cite this article
Renner, S., Sahlén, G. & Périco, E. Testing Dragonflies as Species Richness Indicators in a Fragmented Subtropical Atlantic Forest Environment. Neotrop Entomol 45, 231–239 (2016). https://doi.org/10.1007/s13744-015-0355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-015-0355-9