Abstract
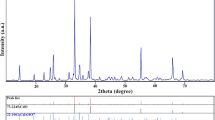
For the first time, bituminous-based mesoporous carbon/zinc oxide (BMC/ZnO) nanocomposite was synthesized and its hydrogen storage capacity was investigated. At first, ZnO nanoparticles were prepared via the homogeneous precipitation technique using green tea extract as a participant agent. In the second step, mesoporous carbon was made from bituminous coal; then, BMC/ZnO nanocomposite was prepared with a facile technique. Detailed investigation of structural and physical properties of the prepared BMC/ZnO nanocomposite was conducted using X-ray diffractometer (XRD), Brunauer–Emmett–Teller (BET), scanning electron microscopy (SEM), and Fourier transform infrared spectrometer (FTIR). Hydrogen storage capacity of produced nanocomposite was inspected using the chronopotentiometry technique. As a result, BMC/ZnO nanocomposite as storage material shows a high electrochemical hydrogen storage equal to 4648 mAh/g. The well-structured, easily prepared and high hydrogen storage performance BMC/ZnO nanocomposite offers hopeful potential to support progressive systems of hydrogen storage.







Similar content being viewed by others
References
H.-M. Cheng, Q.-H. Yang, C. Liu, Hydrogen storage in carbon nanotubes. Carbon 39(10), 1447–1454 (2001)
S. Seifi, S. Masoum, Preparation of copper oxide/oak-based biomass nanocomposite for electrochemical hydrogen storage. Int. J. Hydrogen Energy 44(23), 11979–11988 (2019)
T. Gholami, M. Salavati-Niasari, A. Salehabadi, M. Amiri, M. Shabani-Nooshabadi, M. Rezaie, Electrochemical hydrogen storage properties of NiAl2O4/NiO nanostructures using TiO2, SiO2 and graphene by auto-combustion method using green tea extract. Renew. Energy 115, 199–207 (2018)
A. Salehabadi, M. Salavati-Niasari, F. Sarrami, A. Karton, Sol-Gel auto-combustion synthesis and physicochemical properties of BaAl2O4 nanoparticles; electrochemical hydrogen storage performance and density functional theory. Renew. Energy 114, 1419–1426 (2017)
A. Salehabadi, M.F. Umar, A. Ahmad, M.I. Ahmad, N. Ismail, M. Rafatullah, Carbon-based nanocomposites in solid-state hydrogen storage technology: An overview. Int. J. Energy Res. 44(14), 11044–11058 (2020)
X. Wan, X. Wei, J. Miao, R. Zhang, J. Zhang, Q.J. Niu, Pd-Ni/Cd loaded PPy/Ti composite electrode: synthesis, characterization, and application for hydrogen storage. Int. J. Energy Res. 43(8), 3284–3294 (2019)
A. Eftekhari, B. Fang, Electrochemical hydrogen storage: opportunities for fuel storage, batteries, fuel cells, and supercapacitors. Int. J. Hydrogen Energy 42(40), 25143–25165 (2017)
S. Seifi, S. Masoum, Synthesis of Co9S8@ N, S Co-doped porous carbon core-shell nanocomposite with highly coulombic efficiency in electrochemical hydrogen storage application. J. Electrochem. Soc. 167(11), 110539 (2020)
H. Seifi, S. Masoum, S.A.H. Tafreshi, S. Seifi, S.M. Jafari, Highly porous carbon from microalga, Chlorella Vulgaris, as an electrochemical hydrogen storage material. J. Electrochem. Soc. 167(12), 120525 (2020)
A. Salehabadi, N. Ismail, N. Morad, M. Rafatullah, M. Idayu Ahmad, Preparation and application of sulfonated polysulfone in an electrochemical hydrogen storage system. Int. J. Energy Res. 45(3), 4026–4035 (2021)
V. Berube, G. Radtke, M. Dresselhaus, G. Chen, Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 31(6–7), 637–663 (2007)
J. Ren, H.W. Langmi, B.C. North, M. Mathe, Review on processing of metal–organic framework (MOF) materials towards system integration for hydrogen storage. Int. J. Energy Res. 39(5), 607–620 (2015)
B. Panella, M. Hirscher, Hydrogen physisorption in metal–organic porous crystals. Adv. Mater. 17(5), 538–41 (2005)
C. Strydom, J. Bunt, H. Schobert, M. Raghoo, Changes to the organic functional groups of an inertinite rich medium rank bituminous coal during acid treatment processes. Fuel Process. Technol. 92(4), 764–770 (2011)
W. Xia, J. Yang, C. Liang, Investigation of changes in surface properties of bituminous coal during natural weathering processes by XPS and SEM. Appl. Surf. Sci. 293, 293–298 (2014)
L. Giroux, J.-P. Charland, J.A. MacPhee, Application of thermogravimetric Fourier transform infrared spectroscopy (TG− FTIR) to the analysis of oxygen functional groups in coal. Energy Fuels 20(5), 1988–1996 (2006)
H. Seifi, S. Masoum, Ultrasonically assisted removal of toxic dye using Iranian bituminous coal based-activated carbon: synthesis, characterization, modeling, equilibrium and kinetic studies. J. Iran. Chem. Soc. 17(11), 2969–2980 (2020)
X. Yu, Z. Tang, D. Sun, L. Ouyang, M. Zhu, Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater Sci. 88, 1–48 (2017)
N.N. Sulaiman, M. Ismail, S.N. Timmiati, K.L. Lim, Improved hydrogen storage performances of LiAlH4 + Mg (BH4)2 composite with TiF3 addition. Int. J. Energy Res. 45(2), 2882–2898 (2021)
G. Surucu, A. Gencer, A. Candan, H.H. Gullu, M. Isik, CaXH3 (X= Mn, Fe, Co) perovskite-type hydrides for hydrogen storage applications. Int. J. Energy Res. 44(3), 2345–2354 (2020)
T.M. Chung, Y. Jeong, Q. Chen, A. Kleinhammes, Y. Wu, Synthesis of microporous boron-substituted carbon (B/C) materials using polymeric precursors for hydrogen physisorption. J. Am. Chem. Soc. 130(21), 6668–6669 (2008)
Z. Guan, X. Wang, T. Li, Q. Zhu, M. Jia, B. Xu, Facile synthesis of rutile TiO2/carbon nanosheet composite from MAX phase for lithium storage. J. Mater. Sci. Technol. 35(9), 1977–1981 (2019)
D.I. Abouelamaiem, M.J. Mostazo-López, G. He, D. Patel, T.P. Neville, I.P. Parkin et al., New insights into the electrochemical behaviour of porous carbon electrodes for supercapacitors. J. Energy Storage 19, 337–347 (2018)
Y. Dabaki, S. Boussami, C. Khaldi, H. Takenouti, O. Elkedim, N. Fenineche et al., The effect of ZnO addition on the electrochemical properties of the LaNi3.55Mn0.4Al0.3Co0.2Fe0.55 electrode used in nickel–metal hydride batteries. J. Solid State Electrochem. 21(4), 1157–64 (2017)
X. Chen, S. Jeyaseelan, N. Graham, Physical and chemical properties study of the activated carbon made from sewage sludge. Waste Manage. 22(7), 755–760 (2002)
A. Eftekhari, B. Yazdani, Initiating electropolymerization on graphene sheets in graphite oxide structure. J. Polym. Sci., Part A: Polym. Chem. 48(10), 2204–2213 (2010)
Y.-C. Chiu, C.-L. Huang, C. Wang, Rheological and conductivity percolations of syndiotactic polystyrene composites filled with graphene nanosheets and carbon nanotubes: a comparative study. Compos. Sci. Technol. 134, 153–160 (2016)
X. Zhong, F. Dehghani, Solvent free synthesis of organometallic catalysts for the copolymerisation of carbon dioxide and propylene oxide. Appl. Catal. B 98(3–4), 101–111 (2010)
J. Mu, C. Shao, Z. Guo, Z. Zhang, M. Zhang, P. Zhang et al., High photocatalytic activity of ZnO− carbon nanofiber heteroarchitectures. ACS Appl. Mater. Interfaces. 3(2), 590–596 (2011)
B. Dang, Q. Li, Y. Zhou, J. Hu, J. He, Suppression of elevated temperature space charge accumulation in polypropylene/elastomer blends by deep traps induced by surface-modified ZnO nanoparticles. Compos. Sci. Technol. 153, 103–110 (2017)
C. Bouchelta, M.S. Medjram, O. Bertrand, J.-P. Bellat, Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrol. 82(1), 70–77 (2008)
H. Seifi, S. Masoum, Electrochemical hydrogen storage performance of carbon nanosheets synthesized from bituminous coal. Int. J. Hydrogen Energy 42(51), 30145–30155 (2017)
Y. Shu, K. Li, J. Song, B. Li, C. Tang, Single and competitive adsorption of Cd (II) and Pb (II) from aqueous solution by activated carbon prepared with Salix matsudana Kiodz. Water Sci. Technol. 74(12), 2751–2761 (2016)
A. Eftekhari, P. Jafarkhani, Curly graphene with specious interlayers displaying superior capacity for hydrogen storage. J. Phys. Chem. C 117(48), 25845–25851 (2013)
R.L. McCreery, Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 108(7), 2646–2687 (2008)
C. Zhu, Y. He, Y. Liu, N. Kazantseva, P. Sáha, Q. Cheng, ZnO@ MOF@ PANI core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes. J. Energy Chem. 35, 124–131 (2019)
L. Pan, M.B. Sander, X. Huang, J. Li, M. Smith, E. Bittner et al., Microporous metal organic materials: promising candidates as sorbents for hydrogen storage. J. Am. Chem. Soc. 126(5), 1308–1309 (2004)
A.S. Oberoi, J. Andrews, A.L. Chaffee, L. Ciddor, Hydrogen storage capacity of selected activated carbon electrodes made from brown coal. Int. J. Hydrogen Energy 41(48), 23099–23108 (2016)
T. Gholami, M. Salavati-Niasari, Green facile thermal decomposition synthesis, characterization and electrochemical hydrogen storage characteristics of ZnAl2O4 nanostructure. Int. J. Hydrogen Energy 42(27), 17167–17177 (2017)
B. Zhang, X. Ye, W. Dai, W. Hou, Y. Xie, Biomolecule-assisted synthesis and electrochemical hydrogen storage of porous spongelike Ni3S2 nanostructures grown directly on nickel foils. Chem. -A Eur. J. 12(8), 2337–2342 (2006)
X. Chen, X. Gao, H. Zhang, Z. Zhou, W. Hu, G. Pan et al., Preparation and electrochemical hydrogen storage of boron nitride nanotubes. J. Phys. Chem. B 109(23), 11525–11529 (2005)
K. Jurewicz, E. Frackowiak, F.J.A.P.A. Béguin, Towards the mechanism of electrochemical hydrogen storage in nanostructured carbon materials. Appl. Phys. A 78(7), 981–987 (2004)
M. Konni, A.S. Dadhich, S.B. Mukkamala, Impact of surface modifications on hydrogen uptake by Fe@ f-MWCNTs and Cu@ f-MWCNTs at non-cryogenic temperatures. Int. J. Hydrogen Energy 42(2), 953–959 (2017)
S.A. Needham, G. Wang, K. Konstantinov, Y. Tournayre, Z. Lao, H.-K. Liu, Electrochemical performance of Co3O4–C composite anode materials. Electrochem. Solid-State Lett. 9(7), A315–A319 (2006)
S. Yang, P. Gao, D. Bao, Y. Chen, L. Wang, P. Yang et al., Mechanical ball-milling preparation of mass sandwich-like cobalt–graphene nanocomposites with high electrochemical hydrogen storage ability. J. Mater. Chem. A 1(23), 6731–6735 (2013)
M. Masjedi-Arani, M. Salavati-Niasari, Novel synthesis of Zn2GeO4/graphene nanocomposite for enhanced electrochemical hydrogen storage performance. Int. J. Hydrogen Energy 42(27), 17184–17191 (2017)
M. Masjedi-Arani, M. Salavati-Niasari, Ultrasonic assisted synthesis of a nano-sized Co2SnO4/graphene: a potential material for electrochemical hydrogen storage application. Int. J. Hydrogen Energy 43(9), 4381–4392 (2018)
T. Gholami, M. Salavati-Niasari, M. Sabet, Novel green synthesis of ZnAl2O4 and ZnAl2O4/graphene nanocomposite and comparison of electrochemical hydrogen storage and Coulombic efficiency. J. Clean. Prod. 178, 14–21 (2018)
Acknowledgements
The authors are grateful to the University of Kashan for supporting this work by Grant no. 159181/2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state no apparent competing financial interests or personal relationships that could have influenced this paper's findings.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seifi, H., Masoum, S. A study on electrochemical hydrogen storage performance of bituminous-derived mesoporous carbon/zinc oxide nanocomposite. J IRAN CHEM SOC 20, 3059–3068 (2023). https://doi.org/10.1007/s13738-023-02897-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02897-5