Abstract
A new potentiometric method based on the screen-printed ion-selective electrode (SPISE) was described for the determination of Bi(III) ion in different authentic samples. The novelty is based on, for the first time, the utilization of the cerium zirconium phosphotungstate (CZPT) in a screen-printed electrode (SPE) as a sensing material. In the literature, there is no screen-printed ion-selective electrode for the determination of Bi(III) ion. The influences of the paste composition, different conditioning parameters and foreign ions on the electrode performance were investigated. The reversibility and also response time of the electrode have been studied. The electrode showed a Nernstian response of 18.2 mV decade−1 in the concentration range of 3.3 × 10−7–1 × 10−1 mol. L−1. The electrode was found to be usable within the pH range of 3.5–8.0 and exhibited a fast response time, limit of detection (LOD) (1 × 10−7 mol. L−1), limit of quantification (LOQ) (3.33 × 10−7 mol. L−1), long lifetime and good stability. The matched potential method (MPM) was applied to determine the selectivity coefficient. The isothermal temperature coefficient (dEo/dt) of the electrode was calculated. The electrode was successfully applied for the determination of Bi(III) ion in different authentic samples. By comparing the current results with those obtained using inductively coupled plasma optical emission spectrometry, the nominated Bi(III) screen-printed ion-selective electrode has attained acceptable and efficient performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Along with rising water demand and limited resources, one of the most significant challenges is water quality. The amount and quality of water pose a serious hazard to everyone in the world. Industrial wastewater accumulated from several activities with growing applications accompanied by severe environmental pollution that arises with the discharge of untreated industrial contaminants in water streams. This aqueous pollution has to be controlled by accurate identifications using efficient selective sensors [1,2,3] and finding environmentally friendly techniques for water treatment [4,5,6]; then, the remaining contaminants have to be stabilized [7,8,9,10].
On account of its low cost and comparatively lesser toxicity, Bi is replacing lead (Pb) in different industrial applications. Bismuth salts such as Bi subsalicylate, Bi subnitrate and Bi substrate are used as medicinal products for the treatment of different diseases, ranging from simple bacterial infections, such as syphilis, and protozoal infections to severe gastrointestinal tract disorders, such as peptic gastritis or peptic ulcers.
Bismuth and its compounds are used in a vast variety of applications, such as semiconductors, cosmetic preparations, medicine for the treatment of syphilis, peptic ulcers and dermatological disorders, alloys, metallurgical additives, and in the preparation and recycling of uranium nuclear fuels [11, 12]. As the use of bismuth is increasing, its distribution throughout the environment raises the possibility of coming into contact with organisms. Notably, a number of harmful consequences in humans, including nephropathy, osteoarthropathy, hepatitis and neuropathology, have been linked to bismuth compounds. For the purpose of reducing pollution, bismuth determination is therefore crucial.
Several sensitive methods for measuring bismuth were developed including inductively coupled plasma atomic emission spectrometry (ICP-AES) [13, 14] inductively coupled plasma mass spectrometry (ICP-MS) [15, 16], flame atomic absorption spectrometry (FAAS) [17, 18], hydride generation atomic absorption spectrometry (HGAAS) [19, 20] and spectrophotometry [21]. The advantages of these methods are excellent sensitivity, good selectivity and a wide linear range, but on the other hand, they need tedious procedures for sample preparation or require expensive instruments, so they may be prohibitive to many laboratories. A potentiometric method based on ion-selective electrodes (ISEs) offers great advantages, such as ease of sample preparation, robust in operation, simple procedure, relatively short response time, excellent selectivity, wide linear range and low cost.
There are different types of ion-selective electrodes such as PVC polymer membrane and carbon paste electrodes, but there is no screen-printed electrode for the determination of bismuth ion. A liquid membrane electrode based on the ion-association complex trioctylmethylammonium with tetraiodobismuthate incorporated into a poly(vinyl chloride) matrix was offered [22]. Mahajan et al. [23] constructed ion-selective PVC membrane electrodes (PMEs) and coated graphite membrane electrodes (CGEs) incorporating 1,3,4-trisubstituted-2-azetidinone derivatives as receptors for the selective determination of trace amounts of Bi(III) ions. Liu et al. [24] prepared nanoparticles of bismuth sulfide in the organic phase and dispersed the nanoparticles in a PVC membrane to prepare a bismuth ion-selective electrode. An ion-selective membrane electrode based on tridecylmethylammonium chloride (TDMACl) sensitive to a complex of Bi(III) with ethylenediaminetetraacetate acid [Bi (EDTA)] has been proposed by Kharitonov [25].
A potentiometric sensor was prepared to measure Bi(III) ions using a carbon paste electrode (CPE). Several components were chosen to prepare the best CPE, including graphite powder, a binder, carbon-based nanomaterials as a modifier agent and an ionophore (synthetic Schiff base)[26].
Mostafa et al. [27] studied the construction and general performance characteristics of two potentiometric membrane sensors responsive to bismuth. The sensors are based on the use of ion-association complexes of tetraiodobismate(III) (TIB) anion with polyoxyethylene(10) octyl phenyl ether (1) and tris(1,10-phenanthroline)iron(II) (2) as exchange sites in a carbon paste matrix.
Bi(III) PVC membrane ion-selective electrodes based on two compounds, acylhydrazone and thiosemicarbazone with 1,3,4-thiadiazole, was constructed by Yan et al. [28].
However, no previous studies have conducted the use of screen-printed ion-selective electrode for the determination of Bi ions; also, SPE has many advantages rather than PVC membrane electrode. This work reports on an electrochemical method for sensitive and selective determination of Bi(III) ions in different authentic samples. In this regard, a modified SPE electrode has been prepared using cerium zirconium phosphotungstate (as modifier) and ortho-nitrophenyl octyl ether (as solvent mediator). The developed sensor has some advantages such as simplicity of electrode preparation, wide linear range, low detection limit, durability, sensitivity, selectivity and low cost.
Experimental
Apparatus
Laboratory potential measurements were taken using HANNA PH 211 Microprocessor pH meter. Silver–silver chloride double-junction reference electrode (Metrohm 6.0726.100) in conjugation with different ion-selective electrodes was used. pH measurements were taken using Thermo-Orion, model Orion 3 stars, USA. Before analysis, all glassware used was washed carefully with distilled water and dried in the oven before use.
Reagents and solutions
Chemicals and reagents of analytical grade were purchased for use in this study, and double-distilled water was used throughout this study. Sodium dihydrogen phosphate, sodium tungstate, zirconium oxychloride, ceric ammonium nitrate and sodium tetraphenylborate (NaTPB) were supplied from Aldrich. Bismuth nitrate and other metal ion nitrates were received from Merck. Tricresylphosphate (TCP), dioctylphthalate (DOP), dibutylphthalate (DBP), o-nitrophenyloctylether (o-NPOE) and dioctylsebacate (DOS), sodium dihydrogen phosphate, sodium tungstate, zirconium oxychloride and ceric ammonium nitrate were purchased from Sigma. Relatively high molecular weight polyvinylchloride (PVC) and graphite powder (synthetic 1–2 mm) were supplied from Aldrich. Pharmaceutical drugs (Anusol ointment, Anusol suppositories, DE-NOL 120 mg Tablet, Pepto-Bismol Tablet) were parched from a local pharmacy. The pH of the water samples, tab water (sample 1), freshwater from Nile samples (sample 2), seawater (Baltim area, Kafr El Sheikh (sample 3) and industrial wastewater (sample 4) was adjusted to the proper pH by using HNO3 and NaOH.
Synthesis of cerium zirconium phosphotungstate (CZPT) ionophore
Cerium zirconium phosphotungstate (CZPT) was prepared by the method reported by Preetha and Janardanan [29]. It was synthesized by first preparing 1 M solutions of the reagents, sodium dihydrogen phosphate, sodium tungstate, zirconium oxychloride and ceric ammonium nitrate. A mixture of sodium dihydrogen phosphate solution and sodium tungstate solution is mixed and added with constant stirring to a mixture of zirconium oxychloride solution and ceric ammonium nitrate solution at pH 1. It is kept for 24 h for digestion. It is filtered, washed with deionized water and dried at room temperature. Then it is kept overnight in 1 M HNO3, with occasional shaking and changing the acid for the sake of easy conversion into the H+ form. It is then filtered, washed with deionized water, parched and sieved to obtain particles of uniform size.
Preparation of the Bismuth-modified screen-printed electrodes (SPEs)
Modified SPEs were printed in arrays of six couples consisting of the working and the reference electrodes (each 5 mm × 35 mm) following the procedures previously described [1, 3, 30, 31]. A polyvinyl chloride flexible sheet (0.2 mm) was used as a substrate that was not affected by the curing temperature or the ink solvent and was easily cut by scissors. The working electrodes were prepared depending on the method of fabrication. The working electrode was printed using homemade carbon ink (prepared by mixing 2.5–15 mg (CZPT), 450 mg o-NPOE, 1.25 g of polyvinyl chloride 8% and 0.75 g of carbon powder). They were developed by printing with homemade carbon ink and curing them for 30 min at 50 °C. Then, an insulator layer was sprayed over the printed electrodes, producing a distinct rectangle (5 mm × 5 mm) working area and a second, identical region (for the electrical contact) on the other side. Fabricated electrodes were stored at 4 °C and used directly in the potentiometric measurements.
Calibration of the sensor
The proposed sensor was submerged in a range of standard Bi(III) ion solutions (1 × 10−8–1 × 10−1 mol L−1) in conjunction with the Ag–AgCl reference electrode to generate the calibration curve. The potential readings were portrayed as a function of the concentration of Bi(III) ions as measured by the negative logarithm.
Analytical applications
The developed potentiometric SPE was applied for the determination of Bi(III) ion concentration in pharmaceutical preparation (Anusol ointment, Anusol suppositories, DE-NOL 120 mg Tablet, Pepto-Bismol Tablet). Additionally, in various water samples injected with varying amounts of the ion Bi(III), potential readings of the prepared samples were recorded, and their corresponding concentrations were determined from the calibration plots.
Results and discussion
Polyvinyl chloride (PVC) or liquid-based membrane-based metal-sensitive electrodes were described. The lengthy and inconsistent hand production processes, the challenge of producing small electrodes and the shorter life duration of the electrode were drawbacks of employing polyvinyl chloride membrane electrodes. SPEs have several advantages of mass production, low cost, small size, reproducibility of the preparation process, straightforward, cheap and quick preparation process, as well as the ability for construction of a two-electrode potentiometric strip including both the working and reference electrodes, which provide the possibility of measurements on small volumes as well as the ability for construction of a portable titration system for field titration of metals. Therefore, the goal of this article is the fabrication of novel modified screen-printed electrode for the determination of Bi(III) in different authentic samples. The new electrode was fully characterized according to a previous work published in the International Conference on Harmonization guidelines (ICH) [32]. The primary requirement for an inorganic ion exchanger to be a good electroactive component of membranes is its selective exchange. Keeping this in view, the literature was surveyed and it was found that cerium zirconium phosphotungstate is an inorganic ion exchanger, which shows complete uptake for Bi(III). Thus, this exchanger could be a good material for developing a Bi(III) selective electrode.
Composition and characteristics of the electrodes
The sensitivity of a potentiometric SPE depends on the screen-printed composition. The influence of modifier exchanger amount in the screen-printed was studied. With the other components being the same, six modified SPEs (electrodes I–VI) were created for this purpose. These electrodes each contain 2.5, 5, 7.5, 10, 12.5 and 15 mg of cerium zirconium phosphotungstate. Table 1 lists the resulting slopes and correlation coefficients. These findings demonstrate that electrode V, a modified electrode containing 12.5 mg of modifier exchanger, has a higher Nernstian slope (18.2) and a broad range of linearity (1 × 10−7–1 × 10−1 mol L−1). 12.5 mg modifier exchanger was chosen as the optimum amount for the bismuth electrode fabrication. The electrode surface was renewed before a new set of measurements to get optimal analytical parameters. The surface of a screen-printed electrode can adsorb metal cations from the sample solution. The potentials generated are ascribed to the exchange of Bi(III) species on cation exchanger as is the usual case with all cation exchange membranes. The cation exchange processes between H+ on the surface of the solid and Bi3+ in solution can be represented by the following reaction: \(\overline{{{\text{nH}}^{ + } }} + {\text{Bi}}^{3 + } \leftrightarrow \, \overline{{ {\text{Bi}}^{3 + } }} + {\text{nH}}^{ + }\)
Here the bar over a character denotes the concentration of Bi3+ in sensing material and no bar denotes the concentration of Bi3+ in the solution phase.
When the Bi(III) concentration changed from higher to lower values, worse detection limits and lower linearity ranges were obtained due to this residual Bi ion on the electrode surface. The detection limit was determined conventionally from the intersection of the two extrapolated segments of the calibration graph and found for the proposed sensor × 10−7 mol L−1.
Effect of the plasticizer type
The influence of the plasticizer was investigated since the polarity of the electrode plasticizer might significantly affect the selectivity and dynamic response range of ion-selective electrodes (ISEs). To be utilized in sensors, a suitable plasticizer must possess unique traits including low vapor pressure, high lipophilicity, high molecular weight and a high ability to dissolve the substrate and other additives contained in the matrix.
The plasticizer, a crucial component of the electrode, affects the electroactive cation’s mobility in the ink as well as the electrodes’ detection limit, sensitivity and selectivity. By incorporating plasticizers into the paste, an attempt was made to improve performance. The use of plasticizers enhances the paste’s workability while also greatly enhancing the working concentration range, stability and shelf life of the sensor. The influence of the plasticizer choice on the electrode performances (electrode V) was studied as the electrode plasticized with o-nitrophenyloctylether (o-NPOE) is compared with those plasticized with dioctylsebacate (DOS), dibutylphthalate (DBP), tricresylphosphate (TCP) and dioctylphthalate (DOP) as shown in Table 2. o-NPOE exhibits the biggest total potential change of the five tested plasticizers and the quickest response time when compared to other electrodes, which is reflected in the total time needed to take stable potential measurements. This may be attributed to the fact that o-NPOE has a greater polarity and less lipophilicity that make it more suitable for the screen-printed electrodes [2].
Performance characteristics of the suggested sensor
The potentiometric response characteristics of the bismuth sensor based on the use of CZPT as an electroactive material and o-NPOE as a plasticizer in screen-printed electrode (electrode V) were evaluated according to IUPAC recommendations [33], and the results are given in Table 3. The sensor displays a linear response for 1 × 10−7–1 × 10−1 mol L−1 Bi(III) ion with a Nernstian cationic slope of 18.2 ± 0.73 mV decade−1. The limit of detection (LOD) is 1 × 10−7 mol L−1
Response time
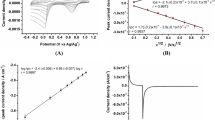
The dynamic response time of the electrode V was tested by measuring the time required to achieve a steady-state potential (within ± 1 mV) after successive immersion of the electrode in a series of bismuth solutions, each having a tenfold increase in concentration from 10−6 to 10−3 mol L−1 of Bi(III) ion (Fig. 1). The SPE (electrode V) shows very fast response time (7 s for concentration 10−3 mol L−1 and 10 s for lower concentration), and the equilibrium potentials essentially remained constant for 7 min. These fast response times can be explained by the fact that this electrode contains carbon particles surrounded by a very thin film of o-NPOE and acting as a conductor and the absence of the internal reference solution.
Lifetime
The average lifetime for most of the reported ion-selective sensors is in the range of 160–200 days. After this time, the slope of the sensor will decrease, and the detection limit will increase [34]. The modified electrode (electrode V) reported herein was tested for a period of 200 days, during which the electrode was used extensively (1 h per day). The modified SPE (electrode V) can be used for 180 days. It is obvious that at first, a slight gradual decrease in the slopes (from 18.20 to 17.95 mV decade−1) (Fig. 2) and, secondly, increases in the detection limit (1 × 10−7–1 × 10−6 mol L−1) were observed. The reason for this limited lifetime of the modified electrode can be attributed to one of the following factors, namely the loss of plasticizer, carrier or ionic site from the polymeric film due to leaching into the sample.
Effect of pH
The effect of pH of the test solutions on the electrode potentials was studied. The pH was adjusted using HCl or NaOH to the test solutions 1 × 10−2 and 1 × 10−3 mol L−1. For each pH value, the potential was recorded, and thus, the potential–pH curves for two Bi(III) concentrations were constructed. This figure shows that the slope per decade concentration is constant ~ 18.20 mV in the pH range 3.5–8.0 (Fig. 3). The decrease in mV readings at pH < 3.5 may be due to interference of hydronium ion. At higher pH values (pH > 8.0), free-base precipitates in the test solution and consequently the concentration of unprotonated species gradually increased. As a result, lower e.m.f. readings were recorded.
Effect of temperature of the test solution
Calibration graphs (electrode potential (Eelec) versus p[Bi(III)]) were constructed at different test solution temperatures (20–60 °C) in order to determine the isothermal coefficient (dEo/dt) of the electrode. The standard electrode potentials (Eo) at the different temperatures were obtained from calibration graphs as the intercepts at \({\text{p}}\left[ {{\text{Bi}}\left( {{\text{III}}} \right)} \right] = 0\) were plotted versus (t − 25), where t was the temperature of the test solution in °C (Fig. 4) where a straight-line plot is obtained according to Antropov’s equation [2].
where Eo(25) is the standard electrode potential at 25 °C. The slope of the straight line obtained represents the isothermal coefficient of the electrode which is found to be 0.0907 V/°C. The value of the obtained isothermal coefficient of the electrode indicates that the electrode has a fairly high thermal stability within the investigated temperature range. The investigated electrode was found to be usable up to 50 °C without noticeable deviation from the Nernstian behavior.
Potentiometric selectivity
“Selectivity” is defined as the response of ion-selective sensors to the target ion, when concomitant ions occur in parallel in the solution. Selectivity coefficients for Bi(III)-SPE toward various inorganic cations were assessed using the “matched potential method” [2]. The selectivity of screen-printed sensors is one of the most important performance parameters that determine the utility of the sensor. As a result, only the sensor (electrode V) that demonstrated the highest performance characteristics in terms of working concentration range, slope, reaction time, and lifetime was used in the selectivity research. A selectivity coefficient with a value of 1.0 shows an equal sensitivity to primary and interfering ions. Table 4 shows that the calculated selectivity coefficients are substantially less than 1.0. Therefore, compared to all other interfering ions investigated and mentioned in Table 4, the electrode is significantly selective to Bi(III) ions. Thus, the selectivity coefficient indicates that it is possible to determine Bi(III) in the presence of interfering ions at a concentration level smaller or slightly higher than the Bi(III) concentration. It is important to point out that the concentration level of the interfering ion, which the electrode can tolerate, depends on the numerical value of selectivity coefficient. The smaller the value of selectivity coefficient, the higher is the concentration of interfering ion(s) which can be tolerated by the sensor. In order to have the practical idea of the concentration level which can be tolerated, the mixed run studies were carried out in the presence of different concentrations of Bi(III), which showed relatively higher values of selectivity coefficient of the electrode. Alkali and alkaline earth metals as well as transition metal ions do not interfere in the electrode response as shown in Table 4.
Analytical applications
To evaluate the applicability of the Bi(III) screen-printed electrode for real samples, electrode V was used for determining the concentration of Bi(III) ions in pharmaceutical drugs and different water samples. The results obtained with the sensor and ICP-OES are summarized in Table 5. Table 5 shows that the obtained results are comparable with those acquired through the ICP-OES analysis of the same samples, which is a good indication of the applicability of the Bi(III) screen-printed electrode for real samples analysis.
Comparison study
For comparative purposes, Table 6 lists the slope, linear range, pH, response time and lifetime of previously published Bi(III)-selective electrodes [2, 13,14,15,16,17,18, 28,29,30,31,32] against the proposed electrode based on cerium zirconium phosphotungstate as ionophore. From the results in this table, it can be concluded that the proposed electrode has long lifetime (24 weeks) with comparing with the previously reported electrodes. Meanwhile, relatively cheap and easily construction indicate that the proposed screen-printed electrode can be used widely in the future.
Method validation
Method validation is the process important to confirm that the analytical procedure used for a specific test is suitable for its intended use [33]. The validation parameters such as robustness/ruggedness, accuracy, precision, specificity and the limit of detection (LOD) were studied using the proposed electrode.
Repeatability/reproducibility
The repeatability of the method has been examined by measuring the potential response of different concentrations of bismuth over a wide time interval of 5 days. The repeatability of the measuring solution was found to be within ± 0.9 mV over 5 days.
Robustness/ruggedness of the electrode
The screen-printed sensor is stable toward mechanical shocks with increasing the temperature up to 50 °C and with the advantage of the renewal of its surface without changing its properties.
Precision and accuracy
To determine the precision of the proposed method, solutions containing two different concentrations of Bi(III) ion were prepared and analyzed in five replicates within the same day to evaluate repeatability (intra-day precision) and over five days to evaluate intermediate precision (inter-day precision). From the data listed in Table 7, the low values of the relative standard deviation (% RSD) indicate the precision of the proposed sensor.
The closeness between the obtained value and the true or accepted reference value expressed about accuracy [31]. The data listed in Table 7 indicated the successful use of the proposed sensor for the determination of Bi(III) ion with high accuracy as indicated by the percentage recovery values.
The lower limit of detection
The lower limit of detection may be taken as the concentration of Bi(III) ion at the point of intersection of the extrapolated linear midrange and final low concentration level segments of the calibration curve [31]. The value obtained indicates that the proposed sensor can detect low concentrations of Bi (Table 3).
Specificity
Specificity is the ability of the method to measure the analyte response in the presence of all the potential interference. The response of the proposed electrode in the presence of interferences was compared with its response for pure Bi(III). It was found that the results obtained by of proposed electrode have unnoticeable interference with the examined species.
Conclusion
A new screen-printed electrode for Bi(III) ions determination based on CZPT as an ionophore was investigated. The use of the new Bi-SPE based on CZPT provides the best response characteristics with Nernstian behavior over a wide concentration range of 1 × 10−7–1 × 10−1 mol L−1 and a fast response time of less than 7 s. The sensor works well in a pH 3.5–8.0. Consequently, the proposed sensor is superior to the existing sensors in terms of response time and lifetime and, on the other hand, comparable with regard to other parameters such as slope, pH range, concentration range and selectivity. The proposed Bi screen-printed electrode has attained an efficient performance in determination of Bi(III) concentration in different real samples such as pharmaceutical drugs and various water samples.
References
R.F. Aglan, H.M. Saleh, G.G. Mohamed, Appl. Water Sci. 8, 141 (2018)
R.F. Aglan, M.M. Hamed, H.M. Saleh, J. Anal. Sci. Technol. 10, 7 (2019)
R. F. Aglan, H. H. Mahmoud, A. M. Rashad, and H. M. Saleh, J. Iran. Chem. Soc. 1 (n.d.).
M. M. A. Dawoud, M. M. Hegazi, H. M. Saleh, and W. K. El Helew, Int. J. Environ. Sci. Technol. 1 (2022).
H. M. Saleh and S. B. Eskander, J. Nucl. Energy Sci. Power Gener. Technol. 9, (2020).
A.A. Abdelhamid, M.H. Badr, R.A. Mohamed, H.M. Saleh, Sustainability 15, 1600 (2023)
H.M. Saleh, in Clay minerals: geological origin, mechanical properties and industrial applications, 1st edn., ed. by L.R. WesleyClays (Nova Science Publishers Inc, New York, 2014), pp.403–415
H.M. Saleh, R.F. Aglan, H.H. Mahmoud, Prog. Nucl. Energy 219, 103178 (2020)
H.M. Saleh, H.R. Moussa, F.A. ElSaied, M. Dawod, T.A. Bayoumi, R.S. Abdel Wahed, Prog. Nucl. Energy 122, 103285 (2020)
S.B. Eskander, H.M. Saleh, M.E. Tawfik, T.A. Bayoumi, Case Stud. Constr. Mater. 15, e00664 (2021)
Z. Xing, J. Wang, S. Zhang, X. Zhang, Talanta 80, 139 (2009)
S. Moyano, R.G. Wuilloud, R.A. Olsina, J.A. Gásquez, L.D. Martinez, Talanta 54, 211 (2001)
H. Karami, M.F. Mousavi, Y. Yamini, M. Shamsipur, Anal. Chim. Acta 509, 89 (2004)
A. Sabarudin, O. Noguchi, M. Oshima, K. Higuchi, S. Motomizu, Microchim. Acta 159, 341 (2007)
C. Xiong, B. Hu, Talanta 81, 578 (2010)
X. Jia, Y. Han, X. Liu, T. Duan, H. Chen, Microchim. Acta 171, 49 (2010)
K. Suvardhan, K.S. Kumar, D. Rekha, B. Jayaraj, G.K. Naidu, P. Chiranjeevi. RETRACTED: Preconcentration and solid-phase extraction of beryllium, lead, nickel, and bismuth from various water samples using 2-propylpiperidine-1-carbodithioate with flame atomic absorption spectrometry (FAAS), Talanta, 68 (3), 735–740 (2006)
S. Şahan, Ş Saçmacı, U. Şahin, A. Ülgen, Ş Kartal, Talanta 80, 2127 (2010)
O. Cankur, N. Ertaş, O.Y. Ataman, J. Anal. At. Spectrom. 17, 603 (2002)
I. Kula, Y. Arslan, S. Bakırdere, S. Titretir, E. Kendüzler, O.Y. Ataman, Talanta 80, 127 (2009)
A.S. Amin, Spectrosc. Lett. 44, 424 (2011)
K. Ohzeki, T. Kambara, J. Electroanal. Chem. Interfacial Electrochem. 88, 85 (1978)
R.K. Mahajan, R.K. Puri, G. Bhargava, M.P. Mahajan, Anal. Lett. 42, 2444 (2009)
L. Liu, L. Wang, H. Yin, Y. Li, X. He, Anal. Lett. 39, 879 (2006)
S.V. Kharitonov, J. Pharm. Biomed. Anal. 30, 181 (2002)
M. Sanati, M. Masrournia, H. Behmadi, A. Beyramabadi, J. Iran. Chem. Soc. 19(8), 3337–3345 (2022)
G.A.-H. Mostafa, A.M. Homoda, Bull. Chem. Soc. Jpn. 81, 257 (2008)
Z. Yan, S. Wang, H. Wang, S. Wu, Mater. Sci. Eng. C 33, 2562 (2013)
B. Preetha, B.C. Janardanan, Res. J. Chem. Sci 4, 43 (2014)
T.A. Ali, G.G. Mohamed, R.F. Aglan, M.A. Mourad, Russ. J. Electrochem. 54, 201 (2018)
E.Y.Z. Frag, R.F. Aglan, H.A. Mohamed, Arab. J. Chem. 12, 388 (2019)
Guideline, ICH Harmonised Tripartite. Validation of analytical procedures: text and methodology. Q2 (R1) 1 (20), 5 (2005)
R.P. Buck, E. Lindner, Pure Appl. Chem. 66, 2527 (1994)
T.A. Ali, A.A. Farag, G.G. Mohamed, J. Ind. Eng. Chem. 20, 2394 (2014)
A.K. Jain, V.K. Gupta, L.P. Singh, U. Khurana, Electroanalysis 9, 1360 (1997)
P.K. Sharma, M. Sudersanan, P.K. Mathur, Ind. J. Chem. Sect. A 39, 472 (2000)
S.V. Kharitonov, Y.M. Kozyreva, V.I. Zarembo, Pharm. Chem. J. 42, 604 (2008)
M.F.S. Teixeira, O. Fatibello-Filho, Int. J. Pharm. 221, 115 (2001)
M.F. de Souza Teixeira, A.Z. Pinto, O. Fatibello-Filho, Talanta 45, 249 (1997)
W. Szczepaniak, M. Ren, Talanta 30, 945 (1983)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors have equal contributions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aglan, R.F., Hamed, M.M. & Saleh, H.M. A new screen-printed electrode for selective determination of bismuth in different authentic samples. J IRAN CHEM SOC 20, 1481–1490 (2023). https://doi.org/10.1007/s13738-023-02771-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02771-4