Abstract
For investigating reduction of side effects of commercial anticancer drugs, such as cisplatin, three new platinum complexes with glycine derivatives and the formula of [Pt(R-amine)2(R-gly)]NO3 were synthesized where R-amine is propylamine, isopentylamine, and tertpentylamine and R-gly is propylglycine, isopentylglycine, and tertpentylglycine, which were characterized by spectroscopic methods, such as ultraviolet–visible, infrared, and proton nuclear magnetic resonance (1H-NMR) spectroscopy. The in-vitro cytotoxicity of these water-soluble complexes and cisplatin as a controller was assessed against MCF-7 cell line by MTT assay. The 50% inhibitory concentration values showed that the inhibitory effect of propyl derivative was better than the other systems. According to solubility of these compounds in water and also comparing the results of assessing adsorption, distribution, metabolism, and excretion, these compounds can be considered as drug-like molecules and oral medication. Given the density functional theory data, such as electronegativity, nucleophilicity, additional electronic charges, and global softness, anticancer properties of the synthesized complexes were almost similar and predominantly more than the cisplatin. DNA binding modes were evaluated by experimental circular dichroism (CD) spectroscopy, and also theoretical study of molecular docking. CD spectra showed a decrease in the intensity of the positive band and an increase in the negative band indicating formation of DNA-complex electrostatic interaction for positively charged synthesized complexes. CD spectra indicated conversion of B-DNA into A-DNA form via electrostatic interaction for positively charged propyl complex at high concentration. Results of studying molecular docking showed that hydrogen bonding is more effective than other interactions, such as electrostatic and covalent interactions, especially for bulky systems. In this regard, glycine derivatives may reduce the side effects of Pt-drugs in treatment of cancer.
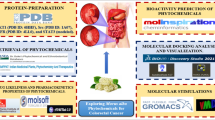
Graphical abstract
Three new anticancer Pt(II) complexes were synthesized with glycine derivatives. In vitro cytotoxicity effects were tested against the human breast cancer cell line of MCF-7. Moreover, the modes of DNA binding with synthesized compounds were investigated using Circular dichroism spectra, ADME prediction, DFT, MEP, and molecular docking.










Similar content being viewed by others
Abbreviations
- MCF-7:
-
Human breast cell line
- Isopentylamine:
-
3-Methyl-butylamine
- Tertpentylamine:
-
1,1-Dimethyl Propylamine
- Gly:
-
Glycine
- CD:
-
Circular dichroism
- MD:
-
Molecular docking
- DFT:
-
Density functional theory
- MEP:
-
Molecular electrostatic potential
- QCDs:
-
Quantum chemical descriptors
- ADME:
-
Adsorption, distribution, metabolism, and excretion
- IC50 :
-
50% Inhibitory concentration
References
F. Safa Shams Abyaneh, M. Eslami Moghadam, M. Hossaini Sadr, A. Divsalar, J. Biomol. Struct. Dyn. 36(4), 893–905 (2018)
W. Tian, L. He, Res. Chem. Intermed. 41, 8725–8733 (2015)
Q.K. Wang, S.P. Pu, Y.N. Li, J. He, L.M. Zhou, J. Peng, T.J. Huang, L.M. Liu, Adv. Mat. Res. 554, 1645–1649 (2012)
Z. Jinchao, L. Li, L. Wang, F. Zhang, X. Li, Eur. J. Med. Chem. 45(11), 5337–5344 (2010)
W. Katarzyna, J. Błasiak, Acta Biochim. Pol. 49(3), 583–596 (2002)
T.J. Sabo, V.M. Dinovic, G.N. Kaluderovic, T.P. Stanojkovic, G.A. Bogdanovic, Z.D. Juranic, Inorganica Chim. Acta. 358, 2239–2245 (2005)
A.R. Khokhar, Y. Deng, S. Al-Baker, M. Yoshida, Z.H. Siddik, J. Inorg. Biochem. 51, 677–687 (1993)
D. Ajloo, M. Eslami Moghadam, K. Ghadimi, M. Ghadamgahi, A.A. Saboury, A. Divsalar, M. SheikhMohammadi, K. Yousefi, Inorganica Chim. Acta. 430, 144–160 (2015)
P. Lunetti, A. Romano, C. Carrisi, D. Antonucci, T. Verri, G.E. De Benedetto, V. Dolce, F.P. Fanizzi, M. Benedetti, L. Capobianco, ChemistrySelect. 1, 4633–4637 (2016)
S. Mukherjee, I. Mitra, P. Das, B. Misini, W. Linert, S.C. Moi, ChemistrySelect. 3, 3871–3885 (2018)
M. Kantoury, M. Eslami Moghadam, A.A. Tarlani, A. Divsalar, Chem. Biol. Drug. Des. 88(1), 76–87 (2016)
S. Shahraki, H. Mansouri-Torshizi, A. Heydari, A. Ghahghaei, A. Divsalar, A.A. Saboury, H. Ghaemi, M. Doostkami, S. Zareian, Iran J. Sci. Technol. Trans. A Sci. 39(2), 187–198 (2015)
A. Muscella, C. Vetrugno, F.P. Fanizzi, C. Manca, S.A. De Pascali, S. Marsigliante, Cell Death Dis. 5(8), e1388 (2014)
I. Mitra, S. Mukherjee, V.P. Reddy, S. Dasgupta, J.C. Bose, S. Mukherjee, W. Linert, SCh. Moi, RSC Adv. 6, 76600–76613 (2016)
K. Choroba, B. Machura, L.R. Raposo, J.G. Małecki, S. Kula, M. Pająk, K. Erfurt, A.M. Maroń, A.R. Fernandes, Dalton Trans. 48(34), 13081–13093 (2019)
S. Hadian Rasanani, M. Eslami Moghadam, E. Soleimani, A. Divsalar, A. Tarlani, J. Coord. Chem. 70, 3769–3789 (2017)
Y.-M. Chang, C.K.-M. Chen, M.-H. Hou, Int. J. Mol. Sci. 13(3), 3394–3413 (2012)
R. D. Dennington II, T.A. Keith, J.M. Millam, GaussView 5.0, Wallingford CT (2009).
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision D.01 (Gaussian Inc, Wallingford CT, 2009).
K. Sayin, S.E. Kariper, M. Taştan, T.A. Sayin, D. Karakaş, J. Mol. Struct. 1176, 478–487 (2019)
T. A. Yousef, S. F. Ahmed, O. A. El-Gammal, G. M. Abu El-Reash, Spectrochim. Acta A Mol. Biomol. Spectrosc. 146, 228–239 (2015)
A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7, 42717 (2017)
A. Divsalar, A.A. Saboury, R. Yousefi, A.A. Moosavi-Movahedi, H. Mansoori-Torshizi, Int. J. Biol. Macromol. 40, 381–386 (2007)
R.A. Condrate, K. Nakamoto, J. Chem. Phys. 42, 2590 (1965)
H. Eshtiagh-Hosseini, M. Mirzaei, M. Biabani, V. Lippolis, M. Chahkandi, C. Bazzicalupi, CrystEngComm 15, 6752–6768 (2013)
E. Efthimiadou, N. Katsaros, A. Karaliota, G. Psomas, Inorg. Chim. Acta 360, 4093–4102 (2007)
K. Sayin, A. Ungordu, Spectrochim. Acta A Mol. Biomol. Spectrosc. 193, 147–155 (2018)
K. Dysz, M. Malik-Gajewska, J. Banach, B. Morzyk-Ociepa, J. Mol. Struct. 1181, 444–454 (2019)
O.A. El-Gammal, T.H. Rakha, H.M. Metwally, G.M. Abu El-Reash, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 127, 144–156 (2014)
S.K. Pandey, S. Pratap, M.K. Tiwari, G. Marverti, J.P. Jasinski, J. Mol. Struct. 1175, 963–970 (2019)
N.R. Sheela, S. Muthu, S. Sampathkrishnan, Spectrochim. Acta A Mol. Biomol. Spectrosc. 120, 237–251 (2014)
S.A. Hosseini, M. Eslami Moghadam, M. Saeidifar, A.A. Saboury, J. Physiol. Pharmacol. 96(12), 1276–1285 (2018)
O.A. Yousif, M.F. Mahdi, A.M.R. Raauf, J. Contemp. Med. Sci. 5(1), 41–50 (2019)
S.P. Singh, N.I. Singh, K. Nongalleima, P. Doley, C.B. Singh, D. Sahoo, Netw. Model Anal. Health Inform. Bioinform. 7, 1 (2018)
Acknowledgments
The authors appreciate being supported by this project by the Chemistry & Chemical Engineering Research Center of Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eslami Moghadam, M., Jafari, A., Kiani Khashandaragh, R. et al. Three anticancer Pt complexes with glycine derivatives: synthesis, bioactivity on MCF-7 cell line, ADME prediction, DFT, MEP, and molecular docking. J IRAN CHEM SOC 18, 1927–1939 (2021). https://doi.org/10.1007/s13738-021-02154-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02154-7