Abstract
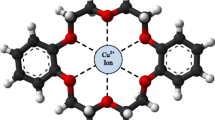
Modified carbon paste electrodes based on amino acid (l-ornithine) or curcumin (turmeric) as ionophores and o-nitrophenyl octyl ether (o-NPOE) as solvent mediator were prepared and investigated as Cu(II) ion-selective electrodes. The reaction mechanism between Cu(II) and l-ornithine (electrode I) or turmeric (electrode II) ionophores at the sensor surface were studied using energy-dispersive X-ray analysis (EDX), scanning electron microscopy (SEM) and IR spectra measurement. These electrodes showed potentiometric response with a divalent cationic Nernstian slopes of 29.0 ± 0.04 and 30.4 ± 0.01 mV decade−1, within a working concentration ranging from 1.0 × 10−6 to 1.0 × 10−2 mol L−1 with a short response time of 10 and 7 s for electrodes I and II, respectively. The electrodes exhibited constant potentiometric response in pH range 2-6 and 3-6 for electrodes I and II, respectively. The proposed sensors exhibited adequate sensitivity to Cu(II) ions over the other metal ions. The sensors were used for estimation of Cu(II) ion concentration in real water samples with satisfactory and good reproducibility results.






Similar content being viewed by others
References
Ö. Dalman, S. Karaböcek, A. Demirak, M. Tüfekçi, Turk. J. Chem. 27, 649 (2003)
J. Škrlíková et al., Microchem. J. 99, 40 (2011)
H.A. Panahi, M. Karimi, E. Moniri, H. Soudi, Afr. J. Pure Appl. Chem. 2, 096 (2008)
V. Kaur, A.K. Malik, Ann. Chim. 97, 1279 (2007)
S. Abbasi, H. Khani, R. Tabaraki, Food Chem. 123, 507 (2010)
B.C. Janegitz et al., Sens. Actuators B Chem. 142, 260 (2009)
rsc.org/Membership/Networking/InterestGroups/Electroanalytical/#, (2012)
H. Nam et al., Proc. IEEE 91, 870 (2003)
A. Lynch et al., Electroanalysis 10, 1096 (1998)
L.R. Bucci, J.F. Hickson, I. Wolinsky, J.M. Pivarnik, Int. J. Sport Nutr. 2, 287 (1992)
R. Kumar, S. Obrai, A. Sharma, A.K. Jassal, M.S. Hundal, J. Mitra, J. Mol. Struct. 1075, 43 (2014)
S. Wanninger, V. Lorenz, A. Subhan, F.T. Edelmann, Chem. Soc. Rev. 44, 4986 (2015)
A. Kareem, Laxmi, M. Arshad, S.A.A. Nami, N. Nishat, J. Photochem. Photobiol. B: Biology. 160, 163 (2016)
K.I. Priyadarsini, Curr. Pharm. Des. 19, 2093 (2013)
L. Nardo, R. Paderno, A. Andreoni, M. Másson, T. Haukvik, H.H. Tønnesen, Spectroscopy. 22, 187 (2008)
M. Călinescu, M. Fiastru, D. Bala, C. Mihailciuc, T. Negreanu-Pîrjol, B. Jurcă, J. Saudi Chem. Soc. 23, 817 (2019)
C.P.V. Mary, S. Vijayakumar, R. Shankar, J. Mol. Graph. Model. 79, 14 (2018)
K. Bairwa, J. Grover, M. Kania, S.M. Jachak, RSC Adv. 4, 13946 (2014)
R.S. Priya, S. Balachandran, J. Daisy, P.V. Mohanan, Univ. J. Phys. Appl. 3, 6 (2015)
D. Pucci, A. Crispini, B.S. Mendiguchia, S. Pirillo, M. Ghedini, S. Morelli, L. De Bartolo, Dalton Trans. 42, 9687 (2013)
E. Ferrari, R. Benassi, S. Sacchi, F. Pignedoli, M. Asti, M. Saladini, J. Inorg. Biochem. 139, 48 (2014)
V.K. Gupta, S. Jain, S. Chandra, Anal. Chim. Acta 486, 199 (2003)
S. Ramaswamy, M. Umadevi, R.K. Rajaram, V. Ramakrishnan, J. Raman Spectrosc. 34, 806 (2003)
C. Conato, A. Contino, G. Maccarrone, A. Magrõ, M. Remelli, G. Tabbõ, Thermochim. Acta 362, 13 (2000)
O. Vajragupta, P. Boonchoong, H. Watanabe, M. Tohda, N. Kummasud, Y. Sumanont, Free Radic. Biol. Med. 35, 1632 (2003)
X. Yuan et al., Anal. Chim. Acta 779, 35 (2013)
E.Y. Frag, R.M. Abdelhameed, Microchem. J. 144, 110 (2019)
E. Bakker, P. Bühlmann, E. Pretsch, Chem. Rev. 97, 3083 (1997)
M.R. Ganjali et al., Sens. Actuators B Chem. 98, 92 (2004)
Antropov, L. Antropov, Theoretical electrochemistry (Mir Publishers, Moscow, 1977)
S. Khalil, S. Abd El-Aliem, J. Pharm. Biomed. Anal. 27, 25 (2002)
E. Bakker, E. Pretsch, P. Bühlmann, Anal. Chem. 72, 1127 (2000)
E. Lindner, Y. Umezawa, Pure Appl. Chem. 80, 85 (2008)
S. Kamata et al., Anal. Chem. 60, 2464 (1988)
Y. Umezawa, K. Umezawa, H. Sato, Pure Appl. Chem. 67, 507 (1995)
M.J. Gismera, M.A. Mendiola, J.R. Procopio, M.T. Sevilla, Anal. Chim. Acta 385, 143 (1999)
M.J. Gismera, D. Hueso, J.R. Procopio, M.T. Sevilla, Anal. Chim. Acta 524, 347 (2004)
M. Ghaedi, S. Naderi, M. Montazerozohori, F. Taghizadeh, A. Asghari, Arab. J. Chem. 10, 2934 (2017)
T.A. Ali, G.G. Mohamed, M.M.I. El-Dessouky, S.M. Abou El-Ella, R.T.F. Mohamed, J. Solut. Chem. 42, 1336 (2013)
E.Y. Frag, M.E. Mohamed, G.G. Mohamed, E.M. Fahim, Appl. Organomet. Chem. 33, 1 (2019)
V.K. Gupta, A.K. Jain, G. Maheshwari, H. Lang, Z. Ishtaiwi, Sens. Actuators B Chem. 117, 99 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frag, E.Y., Mohamed, G.G. & Saad, M. Chemically modified copper potentiometric sensors based on curcumin and amino acid. J IRAN CHEM SOC 18, 651–660 (2021). https://doi.org/10.1007/s13738-020-02051-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02051-5