Abstract
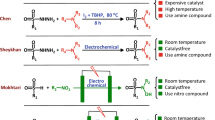
Since derived products from the oxidation of sulfides are vital for pharmaceutical companies, in this paper, electro-oxidation of aryl and alkyl sulfides was investigated on the glassy carbon electrode (GC) by electrochemical techniques such as cyclic voltammetry (CV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS). To choose the best electrolyte, electro-oxidation of organic sulfides was investigated in different solutions such as ethanol, propanol, acetone, acetonitrile, and acetic acid. Among them, acetic acid not only did not have the pesky peak but also produced the largest oxidation current with increasing sulfide. In the following, studies were carried out to optimize the percentage of acetic acid and sodium hydroxide solution as a supporting electrolyte and oxygen source, which eventually a mixture of 85:15 acetic acid and solution of 1 M NaOH had the best oxidation efficiency. It should be noted, this effective media is introduced and reported for the first time. Then, the electro-oxidation of several sulfides were investigated in the optimized electrolyte. Kinetic studies were performed on the introduced system and several kinetic data were determined for methyl phenyl sulfide electrochemical oxidation. Finally, a mechanism for the electro-oxidation of sulfide was proposed.
















Similar content being viewed by others
References
I. Fernandez, N. Khiar, Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 103, 3651–3706 (2003)
W.R.F. Goundry, B. Adams, H. Benson, J. Demeritt, S. McKown, K. Mulholland, A. Robertson, P. Siedlecki, P. Tomlin, K. Vare, Development and scale-up of a biocatalytic process to form a chiral sulfoxide. Org. Process Res. Dev. 21, 107–113 (2017)
E. Hristova, M. Mitov, R. Rashkov, M. Arnaudova, A. Popov, Sulphide oxidation on electrodeposited Ni-Mo-W catalysts. Bulg. Chem. Commun. 40, 291–294 (2008)
B. Miller, A. Chen, Effect of concentration and temperature on electrochemical oscillations during sulfide oxidation on Ti/Ta2O5–IrO2 electrodes. Electrochim. Acta. 50, 2203–2212 (2005)
E. Rafiee, S. Shahebrahimi, Organic-inorganic hybrid polyionic liquid based polyoxometalate as nano porous material for selective oxidation of sulfides. J. Mol. Struct. 1139, 255–263 (2017). https://doi.org/10.1016/j.molstruc.2017.03.041
G. Abdi, A. Alizadeh, M.M. Khodaei, Highly carboxyl-decorated graphene oxide sheets as metal-free catalytic system for chemoselective oxidation of sulfides to sulfones. Mater. Chem. Phys. 201, 323–330 (2017). https://doi.org/10.1016/j.matchemphys.2017.08.062
S. Rayati, E. Khodaei, M. Jafarian, A. Wojtczak, Mn-Schiff base complex supported on magnetic nanoparticles: synthesis, crystal structure, electrochemical properties and catalytic activities for oxidation of olefins and sulfides. Polyhedron 133, 327–335 (2017). https://doi.org/10.1016/j.poly.2017.05.049
S. Rayati, F. Nejabat, S. Zakavi, Chemoselective oxidation of sulfides to sulfoxides with urea hydrogen peroxide (UHP) catalyzed by non-, partially and fully ??-brominated meso-tetraphenylporphyrinatomanganese(III) acetate. Inorg. Chem. Commun. 40, 82–86 (2014). https://doi.org/10.1016/j.inoche.2013.11.036
L.L. Paim, N.R. Stradiotto, Electrooxidation of sulfide by cobalt pentacyanonitrosylferrate film on glassy carbon electrode by cyclic voltammetry. Electrochim. Acta. 55, 4144–4147 (2010). https://doi.org/10.1016/j.electacta.2010.02.082
S. Rayati, E. Khodaei, S. Shokoohi, M. Jafarian, B. Elmi, A. Wojtczak, Cu-Schiff base complex grafted onto graphene oxide nanocomposite: synthesis, crystal structure, electrochemical properties and catalytic activity in oxidation of olefins. Inorganica Chim. Acta. 466, 520–528 (2017)
B. Miller, A. Chen, Effect of concentration and temperature on electrochemical oscillations during sulfide oxidation on Ti/Ta 2 O 5–IrO 2 electrodes. Electrochim. Acta. 50, 2203–2212 (2005)
M. Muñoz, M.A. Gallo, A. Gutiérrez-Alejandre, D. Gazzoli, C.I. Cabello, Molybdenum-containing systems based on natural kaolinite as catalysts for selective oxidation of aromatic sulfides. Appl. Catal. B Environ. 219, 683–692 (2017)
A.J. Bard, L.R. Faulkner, J. Leddy, C.G. Zoski, Electrochemical methods: fundamentals and applications (Wiley, New York, 1980)
G.S. Ferdowsi, S.A. Seyedsadjadi, A. Ghaffarinejad, Ni nanoparticle modified graphite electrode for methanol electrocatalytic oxidation in alkaline media. J. Nanostruct. Chem. 5, 17–23 (2015). https://doi.org/10.1007/s40097-014-0124-z
G.S. Ferdowsi, S.A. Seyedsadjadi, A. Ghaffarinejad, Electroless deposition of the Ni nanoparticles on the graphite electrode for glucose oxidation. Anal. Bioanal. Electrochem. 6, 379–391 (2014)
G.S. Ferdowsi, M. Rahgozar, S.A.S. Sadjadi, A. Ghaffarinejad, Electrocatalytic methanol oxidation with Ni-B and Ni-P electroless modified graphite electrodes. Anal. Bioanal. Electrochem. 11, 774–786 (2019)
L. Dai, Carbon-based catalysts for metal-free electrocatalysis (Curr. Opin, Electrochem, 2017)
O. Hammerich, B. Speiser, Organic electrochemistry: revised and expanded (CRC Press, London, 2015)
B. Zhang, M.-D. Zhou, M. Cokoja, J. Mink, S.-L. Zang, F.E. Kühn, Oxidation of sulfides to sulfoxides mediated by ionic liquids. Rsc Adv. 2, 8416–8420 (2012)
E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 101, 19–28 (1979)
M. Jafarian, M.R. Avei, I. Danaee, F. Gobal, M.G. Mahjani, Electrochemical oxidation of saccharose on copper (hydr) oxide-modified electrode in alkaline media. Chin. J. Catal. 31, 1351–1357 (2010)
A. Ehsani, M. Bigdeloo, F. Asefi, M. Kiamehr, R. Alizadeh, Ternary nanocomposite of conductive polymer/chitosan biopolymer/metal organic framework: synthesis, characterization and electrochemical performance as effective electrode materials in pseudocapacitors. Inorg. Chem. Commun. 115, 107885 (2020)
A. Ehsani, M.G. Mahjani, F. Babaei, H. Mostaanzadeh, Physioelectrochemical and DFT investigation of metal oxide/p-type conductive polymer nanoparticles as an efficient catalyst for the electrocatalytic oxidation of methanol. RSC Adv. 5, 30394–30404 (2015)
A. Ehsani, M.G. Mahjani, M. Jafarian, A. Naeemy, Influence of ionic surfactant on physio-electrochemical properties and fractal dimension of poly ortho aminophenol film. Prog. Org. Coat. 69, 510–516 (2010)
A. Ehsani, M.G. Mahjani, M. Jafarian, A. Naeemy, Electrosynthesis of polypyrrole composite film and electrocatalytic oxidation of ethanol. Electrochim. Acta. 71, 128–133 (2012)
H.M. Shiri, A. Ehsani, Electrosynthesis of neodymium oxide nanorods and its nanocomposite with conjugated conductive polymer as a hybrid electrode material for highly capacitive pseudocapacitors. J. Colloid Interface Sci. 495, 102–110 (2017)
M.G. Mahjani, A. Ehsani, M. Jafarian, Electrochemical study on the semiconductor properties and fractal dimension of poly ortho aminophenol modified graphite electrode in contact with different aqueous electrolytes. Synth. Met. 160, 1252–1258 (2010)
Acknowledgements
We gratefully acknowledge the financial support provided by K. N. Toosi University of Technology Research Council to conduct this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferdowsi, G.S., Jafarian, M. & Khakyzadeh, V. Introducing selective electro-oxidation of aryl and alkyl sulfides protocol in a novel environmentally friendly media. J IRAN CHEM SOC 17, 3009–3018 (2020). https://doi.org/10.1007/s13738-020-01978-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01978-z