Abstract
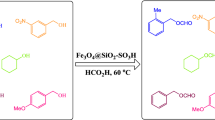
In this study, a novel acid-functionalized magnetic nanoparticles with high loaded multifunctional acidic groups was fabricated by anchoring water-soluble 5-sulfosalicylic acid onto the surface silica-modified Fe3O4. The magnetically recyclable Fe3O4@SiO2@5-SA (20 mg) showed excellent reactivity for greener synthesis of tetrahydrobenzo[b]pyrans via a three-component reaction of different aromatic aldehydes, malononitrile and dimedone in good to excellent yields (70–95%) in pure water at short reaction times (40–150 min). The method shows eco-friendly synthesis of quinoxaline derivatives from direct condensation of substituted 1,2-diamine with various 1,2-dicarbonyl in ethanol at room temperature to afford the desired quinoxalines with good to excellent yields (60–97%) at shorter reaction times (120–240 min). The morphology and magnetic properties of MNPs were studied with scanning electron microscopy, X-ray powder diffraction, Fourier translation infrared spectroscopy, vibrating sample magnetometer and thermogravimetric. The results showed that the Fe3O4@SiO2@5-SA catalyst is completely recoverable by an external magnet and retained catalytic activity after five recycles.










Similar content being viewed by others
References
S. Sadjadi, M.M. Heravi, Methods Org. Synth. 67, 2707 (2011)
Y. Liu, J. Zhang, W. Xu, Curr. Med. Chem. 14, 2872 (2007)
M.M. Heravi, A. Fazeli, Heterocycles 81, 1979 (2010)
C. Liu, B. Pan, Y. Gu, Chin. J. Catal. 37, 979 (2016)
M. Hajjami, F. Gholamian, R.H.E. Hudson, A.M. Sanati, Catal. Lett. 149, 228–247 (2019)
X. Xiong, C. Yi, X. Liao, S. Lai, Catal. Lett. 149, 1690 (2019)
R.Y. Guo, Z.M. An, L.P. Mo, S.X. Wang, Z.H. Zhang, ACS Comb. Sci. 15, 557 (2013)
X.T. Li, Y.H. Liu, X. Liu, Z.H. Zhang, RSC Adv. 5, 25625 (2015)
B.V. Pipaliya, A.K. Chakraborti, ChemCatChem 9, 4191 (2017)
F. Rajabi, D. Alves, R. Luque, Molecules 20, 20709 (2015)
Z. Hajizadeh, A. Maleki, Mol. Catal. 460, 87 (2018)
I. Yavari, M. Bayat, Tetrahedron 59, 2001 (2003)
A.H. Lu, E.L. Salabas, F. Schüth, Angew. Chem. Int. Ed. 46, 1222 (2007)
M.B. Gawande, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42, 3371 (2013)
B.L. Li, H.C. Hu, L.P. Mo, Z.H. Zhang, RSC Adv. 4, 12929–12943 (2014)
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L.V. Elst, R.N. Muller, Chem. Rev. 110, 2574 (2010)
A. Farrokhi, K. Ghodrati, I. Yavari, Catal. Commun. 63, 41 (2015)
V. Azizkhani, F. Montazeri, E. Molashahi, A. Ramazani, J. Energ. Mat. 35, 314 (2017)
J. Deng, L.P. Mo, F.Y. Zhao, L.L. Hou, L. Yang, Z.H. Zhang, Green Chem. 13, 2576 (2011)
A. Maleki, Z. Varzi, F. Hassanzadeh-Afruzi, Polyhedron 171, 193 (2019)
R. Gupta, M. Yadav, R. Gaur, G. Arora, R.K. Sharma, Green Chem. 19, 3801 (2017)
M. Kazemi, M. Mohammadi, Appl. Organomet. Chem. 34, e5400 (2019)
Z. Chen, S. Mohammadi Nasr, M. Kazemi, M. Mohammadi, Mini-Rev. Org. Chem. (2019). https://doi.org/10.2174/1570193X16666190723111746
M. Nikoorazm, M. Khanmoradi, M. Mohammadi, Appl. Organomet. Chem. 34, e5504 (2020)
T. Tamoradi, S.M. Mousavi, M. Mohammadi, New J. Chem. 44, 3012 (2020)
M. Mohammadi, A. Ghorbani-Choghamarani, New J. Chem. 44, 2919 (2020)
A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi, M. Ghadermazi, Polyhedron 158, 25 (2019)
A. Ghorbani-Choghamarani, M. Mohammadi, Z. Taherinia, J. Iran. Chem. Soc. 16, 411 (2019)
L. Chen, A.N. Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
A. Ghorbani-Choghamarani, M. Mohammadi, L. Shiri, Z. Taherinia, Res. Chem. Intermed. 45, 5705 (2019)
D.W.C. MacMillan, Nature 455, 304 (2008)
B. List, Chem. Rev. 107, 5413 (2007)
P.I. Dalko, L. Moisan, Angew. Chem. Int. Ed. 43, 5138 (2004)
R. Mrowczynski, A. Nan, J. Liebscher, RSC Adv. 4, 5927 (2014)
A. Madarasz, Z. Dosa, S. Varga, T. Soos, A. Csampai, I. Papai, ACS Catal. 6, 4379 (2016)
P. Riente, J. Yadav, M.A. Pericàs, Org. Lett. 14, 3668 (2012)
N.G. Singh, M. Lily, S.P. Devi, N. Rahman, A. Ahmed, A.K. Chandra, R. Nongkhlaw, Green Chem. 18, 4216 (2016)
N. Azizi, R. Baghi, H. Ghafuri, M. Bolourtchian, M. Hashemi, Synlett 2010, 379 (2010)
M.R. Saidi, N. Azizi, H. Zali-Boinee, Tetrahedron 57, 6829 (2001)
N. Azizi, M. Mariami, M. Edrisi, Dyes Pigments 100, 215 (2014)
N. Azizi, B. Mirmashhori, M.R. Saidi, Catal. Commun. 8, 2198 (2007)
S. Shinde, G. Rashinkar, R. Salunkhe, J. Mol. Liq. 178, 122 (2013)
Y. Fengping, P. Yanqing, S. Gonghua, Tetrahedron Lett. 46, 3931 (2005)
K. Singh, J. Singh, H. Singh, Tetrahedron 52, 14273 (1996)
K. Abu, T.L. Mohan, A. Shahzad, K.M. Musawwer, Tetrahedron Lett. 52, 5327 (2011)
S. Balalaie, M. Bararjanian, M. Sheikh-Ahmadi, S. Hekmat, P. Salehi, Synth. Commun. 7, 1097 (2007)
D. Tahmassebi, J.A. Bryson, S.I. Binz, Synth. Commun. 41, 2701 (2011)
J.C. Xu, W.M. Li, H. Zheng, L. Yi-Feng, P.F. Zhang, Tetrahedron 67, 9582 (2011)
P. Bhattacharyya, K. Pradhan, S. Paul, A.R. Das, Tetrahedron Lett. 53, 4687 (2012)
M.S. Singh, G. Chandra Nandi, S. Samai, Green Chem. 14, 447 (2012)
M. Honarmand, A. Tzani, D. Anastasi, J. Iran Chem. Soc. 16, 571 (2019)
S. Rostamnia, A. Nuri, H. Xin, A. Pourjavadi, S.H. Hosseini, Tetrahedron Lett. 54, 3344 (2013)
S. Rostamnia, A. Hassankhani, H. Hossieni, H. Golchin, G. Behnam, H. Xin, J. Mol. Catal. A Chem. 395, 463 (2014)
N. Azizi, T.S. Ahooie, M.M. Hashemi, I. Yavari, Synlett 29, 645 (2018)
N.G. Khaligh, T. Mihankhah, Mohd R. Johan, J. Mol. Liq. 277, 794–804 (2019)
Acknowledgements
The financial support of this work provided by CCERCI is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saboury, F., Azizi, N., Mirjafari, Z. et al. A general and inexpensive protocol for the nanomagnetic 5-sulfosalicylic acid catalyzed the synthesis of tetrahydrobenzo[b]pyrans and quinoxaline derivatives. J IRAN CHEM SOC 17, 2533–2543 (2020). https://doi.org/10.1007/s13738-020-01948-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01948-5