Abstract
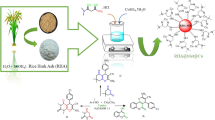
This study aims to use a new medicinal porous plant having a high content of silica known as horsetail and horsetail ash for the first time as novel, efficient, and environmentally friendly natural mild catalysts. The structure of these catalysts was characterized by different techniques such as FT-IR, XRF, SEM–EDS, N2 adsorption–desorption, XRD, and ICP analysis. The results obtained from the analysis revealed that both horsetail and horsetail ash could act as a solid acid catalyst. In addition, a further detailed analysis illustrated that they have a different surface area, porosity, and crystalline structure which can affect their catalytic activities. The synthesis of 2-amino-4H-chromene derivatives was performed via a one-pot three-component condensation of dimedone, malononitrile, and various aromatic aldehydes to compare their catalytic activities under solvent-free conditions. Due to its high porosity and high surface area, horsetail ash yields better results compared to the horsetail itself. FT-IR, mass, 1H-NMR, and 13C-NMR spectroscopies were used to identify the synthesized compounds in this study. An important advantage of this method is the use of these effective natural catalytic systems with characteristics such as low cost, mild reaction conditions, nontoxicity, and reusability which resulted in corresponding products in high to excellent yields and proper reaction times.










Similar content being viewed by others
References
R.C. Cioc, E. Ruijter, R.V.A. Orru, Green Chem. 16, 2958 (2014)
T.J.J. MŘller, R.V.A. Orru, V.A. Chebanov, Y.I. Sakhno, V.E. Saraev, E.A. Muravyova, A.Y. Andrushchenko, S.M. Desenko, V.R. Akhmetova, G.R. Khabibullina, Multi-component Reactions In Heterocyclic Chemistry (Springer, New York, 2011), pp. 31–73
K. Görlitzer, A. Dehne, E. Engler, Arch. Pharm. 316, 264 (1983)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
T. Raj, R.K. Bhatia, R.K. Sharma, V. Gupta, D. Sharma, M.P.S. Ishar, Eur. J. Med. Chem. 44, 3209 (2009)
P. Vats, V. Hadjimitova, K. Yoncheva, A. Kathuria, A. Sharma, K. Chand, A.J. Duraisamy, A.K. Sharma, A.K. Sharma, L. Saso, S.K. Sharma, Med. Chem. Res. 23, 4907 (2014)
M.K. Manion, D. Hockenbery, Cancer Biol. Ther. 2, 104 (2003)
Z.Q. Xu, K. Pupek, W.J. Suling, L. Enache, M.T. Flavin, Bioorg. Med. Chem. 14, 4610 (2006)
E. Mosaddegh, A. Hassankhani, G. Mansouri, E.-J. Chem. 8, 529 (2011)
A. Khazaei, F. Gholami, V. Khakyzadeh, A.R. Moosavi-Zare, J. Afsar, RSC Adv. 5, 14305 (2015)
Y. Ren, W. Zhang, J. Lu, K. Gao, X. Liao, X. Chen, RSC Adv. 5, 79405 (2015)
H.R. Safaei, M. Shekouhy, S. Rahmanpur, A. Shirinfeshan, Green Chem. 14, 1696 (2012)
M.R. Islami, E. Mosaddegh, Phosphorus Sulfur 184, 3134 (2009)
X.-S. Wang, D.-Q. Shi, S.-J. Tu, C.-S. Yao, Synth. Commun. 33, 119 (2003)
S. Balalaie, M. Sheikh-Ahmadi, M. Bararjanian, Catal. Commun. 8, 1724 (2007)
S.-J. Tu, Y. Gao, C. Guo, D. Shi, Z. Lu, Synth. Commun. 32, 2137 (2002)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625 (2004)
S. Tu, H. Jiang, Q. Zhuang, C. Miao, D. Shi, X. Wang, Y. Gao, Chin. J. Org. Chem. 23, 488 (2003)
O.H. Qareaghaj, S. Mashkouri, M.R. Naimi-Jamal, G. Kaupp, RSC Adv. 4, 48191 (2014)
L. Sapei, Characterisation of silica in Equisetum hyemale and its transformation into biomorphous ceramics, pp. 1–158 (2007)
G. Holzhüter, K. Narayanan, T. Gerber, Anal. Bioanal. Chem. 376, 512 (2003)
D. Cloutier, A.K. Watson, Weed Sci. 33, 358 (1985)
F.H.M. Do Monte, J.G. dos Santos, M. Russi, V.M.N.B. Lanziotti, L.K.A.M. Leal, G.M. de Andrade Cunha, Pharmacol. Res. 49, 239 (2004)
H. Oh, D.H. Kim, J.H. Cho, Y.C. Kim, J. Ethnopharmacol. 95, 421 (2004)
H. Mekhfi, M. El Haouari, A. Legssyer, M. Bnouham, M. Aziz, F. Atmani, A. Remmal, A. Ziyyat, J. Ethnopharmacol. 94, 317 (2004)
N.S. Sandhu, S. Kaur, D. Chopra, Asian J. Pharm. Clin. Res. 3, 146 (2010)
S. Soleimani, F.F. Azarbaizani, V. Nejati, Pak. J. Biol. Sci. 10, 4236 (2007)
N. Radulović, G. Stojanović, R. Palić, Phytother. Res. 20, 85 (2006)
M. Veit, C. Weidner, D. Strack, V. Wray, L. Witte, F.C. Czygan, Phytochemistry 31, 3483 (1992)
A. Carnet, C. Petitjean-Freytet, D. Muller, J. Lamaison, Plants Med. Phytother. 25, 32 (1991)
J.G. dos Santos Junior, F.H.M. do Monte, M.M. Blanco, VMdNB Lanziotti, F.D. Maia, L.K. de Almeida Leal, Pharmacol. Biochem. Behav. 81, 593 (2005)
M. Veit, K. Bauer, H. Geiger, F.C. Czygan, Planta Med. 58, 697 (1992)
B. Fabre, B. Geay, P. Beaufils, Plantes Med. Phytother. 26, 190 (1993)
C. Beckert, C. Horn, J.P. Schnitzler, A. Lehning, W. Heller, M. Veit, Phytochemistry 44, 275 (1997)
T. Řezanka, Phytochemistry 47, 1539 (1998)
F. Adam, K. Kandasamy, S. Balakrishnan, J. Colloid Interface Sci. 304, 137 (2006)
B.M. Cherian, L.A. Pothan, T. Nguyen-Chung, G. Mennig, M. Kottaisamy, S. Thomas, J. Agric. Food Chem. 56, 5617 (2008)
X. Sun, F. Xu, R. Sun, P. Fowler, M. Baird, Carbohydr. Res. 340, 97 (2005)
D. An, Y. Guo, Y. Zhu, Z. Wang, Chem. Eng. J. 162, 509 (2010)
L. Sapei, N. Gierlinger, J. Hartmann, R. Nöske, P. Strauch, O. Paris, Anal. Bioanal. Chem. 389, 1249 (2007)
P. Yuan, P.D. Southon, Z. Liu, M.E.R. Green, J.M. Hook, S.J. Antill, C.J. Kepert, J. Phys. Chem. C 112, 15742 (2008)
M. Thommes, Chem. Ing. Tech. 82, 1059 (2010)
N. Yalcin, V. Sevinc, Ceram. Int. 27, 219 (2001)
M.G. Dekamin, M. Eslami, Green Chem. 16, 4914 (2014)
M.A. Zolfigol, N. Bahrami-Nejad, F. Afsharnadery, S. Baghery, J. Mol. Liq. 221, 851 (2016)
A. Rostami, B. Atashkar, H. Gholami, Catal. Commun. 37, 69 (2013)
S. Rostamnia, A. Nuri, H. Xin, A. Pourjavadi, S.H. Hosseini, Tetrahedron Lett. 54, 3344 (2013)
S. Balalaie, M. Bararjanian, M. Sheikh-Ahmadi, S. Hekmat, P. Salehi, Synth. Commun. 37, 1097 (2007)
R.-Y. Guo, Z.-M. An, L.-P. Mo, R.-Z. Wang, H.-X. Liu, S.-X. Wang, Z.-H. Zhang, ACS Comb. Sci. 15, 557 (2013)
M.A. Zolfigol, M. Safaiee, N. Bahrami-Nejad, New J. Chem. 40, 5071 (2016)
M. Amirnejad, M.R. Naimi-Jamal, H. Tourani, H. Ghafuri, Monatsh. Chem. 144, 1219 (2013)
S. Gao, C.H. Tsai, C. Tseng, C.-F. Yao, Tetrahedron 64, 9143 (2008)
P. Sharma, M. Gupta, R. Kant, V.K. Gupta, RSC Adv. 6, 32052 (2016)
B. Sadeghi, A. Hassanabadi, S. Bidaki, J. Chem. Res. 35, 666 (2011)
H. Naeimi, M. Farahnak Zarabi, Appl. Organomet. Chem. 32, e4225 (2018)
S. Nemouchi, R. Boulcina, B. Carboni, A. Debache, C. R. Chim. 15, 394 (2012)
S. Banerjee, A. Horn, H. Khatri, G. Sereda, Tetrahedron Lett. 52, 1878 (2011)
V. Safarifard, S. Beheshti, A. Morsali, CrystEngCommun 17, 1680 (2015)
S. Pradhan, V. Sahu, B.G. Mishra, J. Mol. Catal. A Chem. 425, 297 (2016)
A. Maleki, R. Ghalavand, R. Firouzi Haji, Appl. Organomet. Chem. 32, e3916 (2018)
Y. Sarrafi, E. Mehrasbi, A. Vahid, M. Tajbakhsh, Chin. J. Catal. 33, 1486 (2012)
K. Niknam, M. Khataminejad, F. Zeyaei, Tetrahedron Lett. 57, 361 (2016)
A. Hasaninejad, M. Shekouhy, N. Golzar, A. Zare, M.M. Doroodmand, Appl. Catal. A Gen. 402, 11 (2011)
M. Abdollahi-Alibeik, F. Nezampour, React. Kinet. Mech. Catal. 108, 213 (2013)
R.S. Bhosale, C.V. Magar, K.S. Solanke, S.B. Mane, S.S. Choudhary, R.P. Pawar, Synth. Commun. 37, 4353 (2007)
B. Maleki, S.S. Ashrafi, RSC Adv. 4, 42873 (2014)
D. Azarifar, S.M. Khatami, M.A. Zolfigol, R. Nejat-Yami, J. Iran. Chem. Soc. 11, 1223 (2014)
R. Heydari, R. Rahimi, M. Kangani, A. Yazdani-Elah-Abadi, M. Lashkari, Acta Chem. Iasi 25, 163 (2017)
A.A. Mohammadi, M.R. Asghariganjeh, A. Hadadzahmatkesh, Arab. J. Chem. 10, S2213 (2017)
P. Bhattacharyya, K. Pradhan, S. Paul, A.R. Das, Tetrahedron Lett. 53, 4687 (2012)
Acknowledgements
We are thankful to Ferdowsi University of Mashhad Research Council for financial support to this work (Grant No: 3/45801).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosseini Mohtasham, N., Gholizadeh, M. Horsetail plant (Equisetum arvense) and horsetail plant ash: application and comparison of their catalytic activities as novel and natural porous lewis acid catalysts for the one-pot green synthesis of 2-amino-4H-chromene derivatives under solvent-free conditions. J IRAN CHEM SOC 17, 397–409 (2020). https://doi.org/10.1007/s13738-019-01777-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01777-1