Abstract
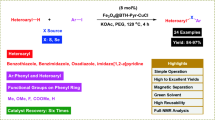
A Cu(II)–Schiff base complex containing imidazolium ionic phase was prepared and decorated on γ-Fe2O3 magnetic nanoparticles (γ-Fe2O3@Cu(II)IL-SB) and found to be an efficient catalyst for the Pd- and base-free Sonogashira coupling reaction. The heterogeneous catalyst was characterized by FTIR spectroscopy, UV–visible spectroscopy, FE-SEM, TEM, XRD spectroscopy, EDX spectroscopy, VSM, ICP spectroscopy, and atomic absorption spectroscopy. The coupling reactions were performed using the catalyst under mild and base-free conditions, and high-to-excellent yields were obtained for a variety of substrates. The catalyst demonstrates a dual-functionality arising from metal sites and imidazolium moieties and that the later plays a base role. Reusability and stability of γ-Fe2O3@Cu(II)IL-SB were studied several times, which can be reused up to eight consecutive runs with at least reduction in catalytic activity. Also, the mechanism of this bifunctional catalytic system was thoroughly investigated.
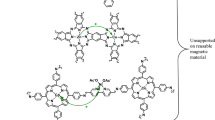
Graphic abstract
A new and efficient method has been developed for the base- and Pd-free Sonogashira cross-coupling reactions of aryl halides with phenyl acetylene using a bifunctional γ-Fe2O3@Cu(II)IL-SB catalyst with imidazolium moiety under mild conditions












Similar content being viewed by others
References
K. Sonogashira, Y. Tohda, N. Hagihara, Tetrahedron Lett. 16, 4467 (1975)
K. Sonogashira, J. Organomet. Chem. 653, 46 (2002)
J. Tan, Y. Bai, X. Zhang, C. Huang, D. Liu, L. Zhang, Macromol. Rapid Commun. 37, 1434 (2016)
M. Altmann, U.H. Bunz, Macromol. Rapid Commun. 15, 785 (1994)
C. Jung, M. Krumova, S. Mecking, Langmuir 30, 9905 (2014)
H. Khojasteh, V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, J. Iran. Chem. Soc. 14, 1139 (2017)
R. Chinchilla, C. Nájera, Chem. Soc. Rev. 40, 5084 (2011)
S.E. Allen, R.R. Walvoord, R. Padilla-Salinas, M.C. Kozlowski, Chem. Rev. 113, 6234 (2013)
I.P. Beletskaya, A.V. Cheprakov, Coord. Chem. Rev. 248, 2337 (2004)
F. Monnier, F. Turtaut, L. Duroure, M. Taillefer, Org. Lett. 10, 3203 (2008)
Z. Wang, T. Zheng, H. Sun, X. Li, O. Fuhr, D. Fenske, New J. Chem. 42, 11465 (2018)
E. Tan, O. Quinonero, M. Elena de Orbe, A.M. Echavarren, ACS Catal. 8, 2166 (2018)
A.P. Thankachan, K.S. Sindhu, S.M. Ujwaldev, G. Anilkumar, Tetrahedron Lett. 58, 536 (2017)
A.R. Hajipour, F. Rezaei, Z. Khorsandi, Green Chem. 19, 1353 (2017)
A.R. Hajipour, S.H. Nazemzadeh, F. Mohammadsaleh, Tetrahedron Lett. 55, 654 (2014)
J.H. Li, J.L. Li, D.P. Wang, S.F. Pi, Y.X. Xie, M.B. Zhang, X.C. Hu, J. Org. Chem. 72, 2053 (2007)
L. Yu, X. Jiang, L. Wang, Z. Li, D. Wu, X. Zhou, Eur. J. Org. Chem. 2010, 5560 (2010)
K.G. Thakur, E.A. Jaseer, A.B. Naidu, G. Sekar, Tetrahedron Lett. 50, 2865 (2009)
H. Oka, K. Kitai, T. Suzuki, Y. Obora, RSC Adv. 7, 22869 (2017)
A.R. Hajipour, S.M. Hosseini, F. Mohammadsaleh, New J. Chem. 40, 6939 (2016)
V. Polshettiwar, R.S. Varma, Green Chem. 12, 743 (2010)
V. Polshettiwar, C. Len, A. Fihri, Coord. Chem. Rev. 253, 2599 (2009)
F. Alonso, M. Yus, ACS Catal. 2, 1441 (2012)
A.H. Latham, M.E. Williams, Acc. Chem. Res. 41, 411 (2008)
K. Niknam, A. Deris, F. Panahi, M.R.H. Nezhad, J. Iran. Chem. Soc. 10, 1291 (2013)
M. Esmaeilpour, J. Javidi, F.N. Dodeji, H. Hassannezhad, J. Iran. Chem. Soc. 11, 1703 (2014)
A. Bee, R. Massart, S. Deveus, J. Magn. Magn. Mater. 149, 6 (1995)
B.Z. Tang, Y. Geng, J.W.Y. Lam, B. Li, X. Jing, X. Wang, X.X. Zhang, Chem. Mater. 11, 1581 (1999)
N.V. Plechkova, K.R. Seddon, Chem. Soc. Rev. 37, 123 (2008)
T. Welton, Chem. Rev. 99, 2071 (1999)
J.P. Hallett, T. Welton, Chem. Rev. 111, 3508 (2011)
W. Zheng, R. Tan, S. Yin, Y. Zhang, G. Zhao, Y. Chen, D. Yin, Catal. Sci. Technol. 5, 2092 (2015)
A. Corma, H. García, A. Leyva, Tetrahedron 60, 8553 (2004)
A.S. Reddy, K.K. Laali, Tetrahedron Lett. 56, 4807 (2015)
J.C. Xiao, B. Twamley, J.N.M. Shreeve, Org. Lett. 6, 3845 (2004)
P. Nehra, B. Khungar, K. Pericherla, S.C. Sivasubramanian, A. Kumar, Green Chem. 16, 4266 (2014)
M. Kazemnejadi, A. Shakeri, M. Mohammadi, M. Tabefam, J. Iran. Chem. Soc. 14, 1917 (2017)
M. Kazemnejadi, A. Shakeri, M. Nikookar, M. Mohammadi, M. Esmaeilpour, Res. Chem. Intermed. 43, 6889 (2017)
M. Kazemnejadi, A. Shakeri, M. Nikookar, R. Shademani, M. Mohammadi, Soc. Open Sci. 5, 171541 (2018)
Y. Peng, Y. Cai, G. Song, J. Chen, Synlett 2005, 2147 (2005)
A.G. Roca, J.F. Marco, M.D.P. Morales, C.J. Serna, J. Phys. Chem. C 111, 18577 (2007)
M. Kazemnejadi, A.R. Sardarian, RSC Adv. 6, 91999 (2016)
Z. Arabpoor, H.R. Shaterian, RSC Adv. 6, 44459 (2016)
L.H. Abdel-Rahman, A.M. Abu-Dief, M. Ismael, M.A. Mohamed, N.A. Hashem, J. Mol. Struct. 1103, 232 (2016)
Z. Li, S. Wu, H. Ding, D. Zheng, J. Hu, X. Wang, Q. Kan, New J. Chem. 37, 1561 (2013)
M. Sarkheil, M. Lashanizadegan, Appl. Organomet. Chem. 31, e3726 (2017)
M. Kazemnejadi, M. Nikookar, M. Mohammadi, A. Shakeri, M. Esmaeilpour, J. Colloid Interface Sci. 527, 298 (2018)
K.M. Ho, P. Li, Langmuir 24, 1801 (2008)
R. Chinchilla, C. Nájera, Chem. Soc. Rev. 40, 5084 (2011)
A.M. Thomas, A. Sujatha, G. Anilkumar, RSC Adv. 4, 21688 (2014)
S. Navalon, M. Alvaro, H. Garcia, ChemCatChem 5, 3460 (2013)
M. Parasram, V. Gevorgyan, Chem. Soc. Rev. 46, 6227 (2017)
R.H. Fath, S.J. Hoseini, New J. Chem. 41, 3392 (2017)
M. Kazemnejadi, Z. Rezazadeh, M.A. Nasseri, A. Allahresani, M. Esmaeilpour, Green Chem. 21, 1718 (2019)
A.S. Reddy, K.K. Laali, Tetrahedron Lett. 56, 4807 (2015)
A. Dewan, M. Sarmah, U. Bora, A.J. Thakur, Tetrahedron Lett. 57, 3760 (2016)
Acknowledgements
The authors are grateful to the University of Birjand for its financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nasseri, M.A., Rezazadeh, Z., Kazemnejadi, M. et al. Magnetic Cu–Schiff base complex with an ionic tail as a recyclable bifunctional catalyst for base/Pd-free Sonogashira coupling reaction. J IRAN CHEM SOC 16, 2693–2705 (2019). https://doi.org/10.1007/s13738-019-01732-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01732-0