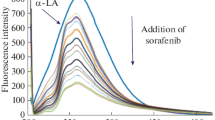
Abstract
The interaction between α-lactalbumin (a-LA) with resveratrol (RES) and curcumin (CUR) was studied by multiple spectroscopic as well as the zeta potential and molecular modeling techniques. Fluorescence spectroscopy showed that RES could quench the a-LA fluorescence with a static mechanism and this quenching effect became more significant when both CUR and RES coexisted. The synchronized fluorescence spectroscopy indicated that the conformation of a-LA and the distance between RES and Trp residues was altered. On the other hand, in the binary system, the RES site was closer to Trp than to Tyr, which proved the significant contribution of the Trp residues to the fluorescence of a-LA. Using red edge excitation shift, we found that the mobility restriction around the Trp residues was unchanged, demonstrating that the rigidity of the environment of Trp residue remained the same. The binding distance between RES and the Trp residues of a-LA was obtained according to Förster’s theory of nonradioactive energy transfer and was found to be 2.67 nm and 2.78 nm for the binary and ternary systems, respectively. This confirmed the existence of static quenching for the binary and ternary systems alike. The quantitative analysis data from the circular dichroism spectra demonstrated that the binding of RES to a-LA induced conformational changes in a-LA. Moreover, the α-helix content in a-LA greatly decreased in the presence of RES and in the ternary system this decrease was significant. Protein–ligand docking suggested that the affinity of RES to the (a-LA–CUR) complex was lower than for RES to a-LA.










Similar content being viewed by others
References
B. Burstein, A. Maguy, R. Clément, H. Gosselin, F. Poulin, N. Ethier, J.-C. Tardif, T.E. Hébert, A. Calderone, S. Nattel, Effects of resveratrol (trans-3, 5, 4′-trihydroxystilbene) treatment on cardiac remodeling following myocardial infarction. J. Pharmacol. Exp. Ther. 323(3), 916–923 (2007)
M. Okawara, H. Katsuki, E. Kurimoto, H. Shibata, T. Kume, A. Akaike, Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol 73(4), 550–560 (2007)
S. Pervaiz, A.L. Holme, Resveratrol: its biologic targets and functional activity. Antioxid. Redox Signal. 11(11), 2851–2897 (2009)
F.Z. Marques, M.A. Markus, B.J. Morris, Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 41(11), 2125–2128 (2009)
M. Mutoh, M. Takahashi, K. Fukuda, Y. Matsushima-Hibiya, H. Mutoh, T. Sugimura, K. Wakabayashi, Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 21(5), 959–963 (2000)
C.R. Pace-Asciak, O. Rounova, S.E. Hahn, E.P. Diamandis, D.M. Goldberg, Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin. Chim. Acta 246(1–2), 163–182 (1996)
J.L. Kipp, V.D. Ramirez, Effect of estradiol, diethylstilbestrol, and resveratrol, on F0F1-ATPase activity from mitochondrial preparations of rat heart, liver, and brain. Endocr. Basingstoke 15(2), 165–176 (2001)
P.S. Ray, G. Maulik, G.A. Cordis, A.A. Bertelli, A. Bertelli, D.K. Das, The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 27(1), 160–169 (1999)
H.P. Ammon, M.A. Wahl, Pharmacology of Curcuma longa. Planta Medica 57(01), 1–7 (1991)
S. Barthelemy, L. Vergnes, M. Moynier, D. Guyot, S. Labidalle, E. Bahraoui, Curcumin and curcumin derivatives inhibit Tat-mediated transactivation of type 1 human immunodeficiency virus long terminal repeat. Res. Virol. 149(1), 43–52 (1998)
M. Ramırez-Tortosa, M. Mesa, M. Aguilera, J. Quiles, L. Baro, C. Ramirez-Tortosa, E. Martinez-Victoria, A. Gil, Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis 147(2), 371–378 (1999)
P. Anand, C. Sundaram, S. Jhurani, A.B. Kunnumakkara, B.B. Aggarwal, Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 267(1), 133–164 (2008)
H. Hatcher, R. Planalp, J. Cho, F. Torti, S. Torti, Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 65(11), 1631–1652 (2008)
A. Khar, A.M. Ali, B. Pardhasaradhi, Z. Begum, R. Anjum, Antitumor activity of curcumin is mediated through the induction of apoptosis in AK-5 tumor cells. FEBS Lett. 445(1), 165–168 (1999)
H. Maier, J. Ilich, J. Kim, M. Spicer, Nutrition supplementation for diabetic wound healing: a systematic review of current literature. Skinmed 11(4), 217–224 (2012) (quiz 224–215)
A. Belatik, C. Kanakis, S. Hotchandani, P. Tarantilis, M. Polissiou, H. Tajmir-Riahi, Locating the binding sites of retinol and retinoic acid with milk β-lactoglobulin. J. Biomol. Struct. Dyn. 30(4), 437–447 (2012)
R.J. FitzGerald, B.A. Murray, D.J. Walsh, Hypotensive peptides from milk proteins. J. Nutr. 134(4), 980S–988S (2004)
K. Acharya, D. Stuart, N. Walker, M. Lewis, D. Phillips, Refined structure of baboon α-lactalbumin at 1.7 Å resolution: comparison with C-type lysozyme. J. Mol. Biol. 208(1), 99–127 (1989)
B.S. Horton, Commercial utilization of minor milk components in the health and food industries. J. Dairy Sci. 78(11), 2584–2589 (1995)
M. Svensson, H. Sabharwal, A. Håkansson, A.-K. Mossberg, P. Lipniunas, H. Leffler, C. Svanborg, S. Linse, Molecular characterization of α-lactalbumin folding variants that induce apoptosis in tumor cells. J. Biol. Chem. 274(10), 6388–6396 (1999)
C.R. Markus, B. Olivier, G.E. Panhuysen, J. Van der Gugten, M.S. Alles, A. Tuiten, H.G. Westenberg, D. Fekkes, H.F. Koppeschaar, E.E. de Haan, The bovine protein α-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am. J. Clin. Nutr. 71(6), 1536–1544 (2000)
H. Teschemacher, G. Koch, V. Brantl, Milk protein-derived opioid receptor ligands. Pept. Sci. 43(2), 99–117 (1997)
L.G. Sternhagen, J.C. Allen, Growth rates of a human colon adenocarcinoma cell line are regulated by the milk protein alpha-lactalbumin, in Bioactive Components of Human Milk, ed. by D.S. Newburg (Springer, Boston, 2001), pp. 115–120
Z. Omidvar, K. Parivar, H. Sanee, Z. Amiri-Tehranizadeh, A. Baratian, M.R. Saberi, A. Asoodeh, J. Chamani, Investigations with spectroscopy, zeta potential and molecular modeling of the non-cooperative behaviour between cyclophosphamide hydrochloride and aspirin upon interaction with human serum albumin: binary and ternary systems from the view point of multi-drug therapy. J. Biomol. Struct. Dyn. 29(1), 181–206 (2011)
J.R. Lakowicz, Plasmonics in biology and plasmon-controlled fluorescence. Plasmonics 1(1), 5–33 (2006)
Z.-D. Qi, B. Zhou, X. Qi, S. Chuan, Y. Liu, J. Dai, Interaction of rofecoxib with human serum albumin: determination of binding constants and the binding site by spectroscopic methods. J. Photochem. Photobiol., A 193(2), 81–88 (2008)
M. Borisover, Y. Laor, N. Bukhanovsky, I. Saadi, Fluorescence-based evidence for adsorptive binding of pyrene to effluent dissolved organic matter. Chemosphere 65(11), 1925–1934 (2006)
J. Równicka-Zubik, A. Sułkowska, J. Pożycka, K. Gaździcka, B. Bojko, M. Maciążek-Jurczyk, W. Sułkowski, Fluorescence analysis of sulfasalazine bound to defatted serum albumin in the presence of denaturating factors. J. Mol. Struct. 924, 371–377 (2009)
M. Ranjan, P. Diffley, G. Stephen, D. Price, T.J. Walton, R.P. Newton, Comparative study of human steroid 5α-reductase isoforms in prostate and female breast skin tissues: sensitivity to inhibition by finasteride and epristeride. Life Sci. 71(2), 115–126 (2002)
T. Wang, B. Xiang, Y. Wang, C. Chen, Y. Dong, H. Fang, M. Wang, Spectroscopic investigation on the binding of bioactive pyridazinone derivative to human serum albumin and molecular modeling. Colloids Surf. B 65(1), 113–119 (2008)
J. Lloyd, Synchronized excitation of fluorescence emission spectra. Nature 231(20), 64–65 (1971)
F. Ding, J. Huang, J. Lin, Z. Li, F. Liu, Z. Jiang, Y. Sun, A study of the binding of CI Mordant Red 3 with bovine serum albumin using fluorescence spectroscopy. Dyes Pigm. 82(1), 65–70 (2009)
H.-M. Zhang, Y.-Q. Wang, M.-L. Jiang, A fluorimetric study of the interaction of CI solvent red 24 with haemoglobin. Dyes Pigm. 82(2), 156–163 (2009)
M. Rodrıguez-Cuesta, R. Boqué, F. Rius, D.P. Zamora, M.M. Galera, A.G. Frenich, Determination of carbendazim, fuberidazole and thiabendazole by three-dimensional excitation–emission matrix fluorescence and parallel factor analysis. Anal. Chim. Acta 491(1), 47–56 (2003)
L. Stryer, Fluorescence energy transfer as a spectroscopic ruler. Ann. Rev. Biochem. 47(1), 819–846 (1978)
F.-Y. Wu, L.-N. Zhang, Z.-J. Ji, X.-F. Wan, Spectroscopic investigation of the interaction between thiourea-zinc complex and serum albumin. J. Lumin. 130(7), 1280–1284 (2010)
G.D. Fasman, Circular dichroism and the conformational analysis of biomolecules (Springer, Berlin, 2013)
P. Yang, F. Gao, The principle of bioinorganic chemistry. Science, Beijing 349 (2002)
J. Kang, Y. Liu, M.-X. Xie, S. Li, M. Jiang, Y.-D. Wang, Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta (BBA) Gen. Subj. 1674(2), 205–214 (2004)
M.C. Tory, A.R. Merrill, Determination of membrane protein topology by red-edge excitation shift analysis: application to the membrane-bound colicin E1 channel peptide. Biochim. Biophys. Acta (BBA) Biomembr. 1564(2), 435–448 (2002)
D. Gao, Y. Tian, F. Liang, D. Jin, Y. Chen, H. Zhang, A. Yu, Investigation on the pH-dependent binding of Eosin Y and bovine serum albumin by spectral methods. J. Lumin. 127(2), 515–522 (2007)
Z. Chen, J. Liu, Y. Han, Rapid and sensitive determination of proteins by enhanced resonance light scattering spectroscopy of sodium lauroyl glutamate. Talanta 71(3), 1246–1251 (2007)
X. Long, C. Zhang, J. Cheng, S. Bi, A novel method for study of the aggregation of protein induced by metal ion aluminum(III) using resonance Rayleigh scattering technique. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 69(1), 71–77 (2008)
Acknowledgements
The financial support of the Research Council of the Mashhad Branch, Islamic Azad University, is gratefully acknowledged. The authors thank Dr. Ljungberg for the English editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jahanshahtalab, M., Kamshad, M., Rezaei, S. et al. New insights into alpha-lactalbumin behavior upon interaction with resveratrol and curcumin by spectroscopic and molecular modeling techniques: binary and ternary system comparison. J IRAN CHEM SOC 16, 1311–1326 (2019). https://doi.org/10.1007/s13738-019-01608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01608-3