Abstract
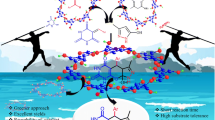
A remarkably simple synthetic method has been described for the access of structurally diverse 3,4-dihydropyrimidin-2(1H)-ones in excellent yields in the presence of 2,4,6-trichloro-1,3,5-triazine (TCT) as an efficient source of hydrochloric acid under ultrasound radiation. In this tandem reaction, a range of aldehydes, β-diketo esters and urea were made to condense together via one-pot, solvent-free synthetic strategy. Use of metal-free catalyst, readily accessible substrates, high production rate and ease of work-up are the imperative features of this protocol.
Graphical abstract






Similar content being viewed by others
References
S. Tang, Y.Y. Si, Z.P. Wang, K.R. Mei, X. Chen, J.Y. Cheng, J.S. Zheng, L. Liu, Angew. Chem. Int. Ed. 54, 5713 (2015)
T. Yatabe, X. Jin, K. Yamaguchi, N. Mizuno, Angew. Chem. Int. Ed. 54, 13302 (2015)
Y. Deng, W. Gong, J. He, J.Q. Yu, Angew. Chem. Int. Ed. 53, 6692 (2014)
M. Yoshida, T. Mizuguchi, K. Namba, Angew. Chem. Int. Ed. 53, 14550 (2014)
A. Nefzi, J.M. Ostresh, R.A. Houghten, Chem. Rev. 97, 449 (1997)
T.L. Ho, Tandem Organic Reactions. Wiley: New York (1992)
A. Agarwal, K. Srivastava, S.K. Puri, P.M.S. Chauhan, Bioorg. Med. Chem. Lett. 15, 531 (2005)
S.B. Katiyar, K. Srivastava, S.K. Puri, P.M.S. Chauhan, Bioorg. Med. Chem. Lett. 15, 4957 (2005)
M.A. Bigdeli, M.M. Heravi, G.H. Mahdavinia, Catal. Commun. 8, 1595 (2007)
L.D. Luca, G. Giacomelli, A. Porcheddu, J. Org. Chem. 67, 6272 (2002)
G.V.M. Sharma, J.J. Reddy, P.S. Lakshmi, Tetrahedron Lett. 45, 7729 (2004)
G.V.M. Sharma, K.L. Reddy, P.S. Lakshmi, P.R. Krishna, Synthesis 2006, 55 (2006)
P. Sivaguru, P. Theerthagiri, A. Lalitha, Tetrahedron Lett. 56, 2203 (2015)
R. Ramesh, S. Maheswari, M. Arivazhagan, J.G. Malecki, A. Lalitha, Tetrahedron Lett. 58, 3905 (2017)
M. Sharma, S. Pandey, K. Chauhan, D. Sharma, B. Kumar, P.M.S. Chauhan, J. Org. Chem. 77, 929 (2012)
K. Harikumar, V. Rajendran, Ultrason. Sonochem 21, 208 (2014)
M. Nasrollahzadeh, A. Ehsani, A.R. Vartouni, Ultrason. Sonochem 21, 275 (2014)
R. Kuppa, V.S. Moholkar, Ultrason. Sonochem. 17, 123 (2010)
C.O. Kappe, J. Org. Chem. 62, 3109 (1997)
F. Bossert, W. Vater, Med. Res. Rev. 9, 291 (1989)
I.T. Phucho, A. Nongpiur, S. Tumtin, R. Nongrum, R.L. Nongkhlaw, Rasayan J. Chem. 2, 662 (2009)
K.I. Sakata, M. Someya, Y. Matsumoto, H. Tauchi, M. Kai, M. Toyota, M. Takagi, M. Hareyama, M. Fukushima, Cancer Sci. 102, 1712 (2011)
A.D. Patil, N.V. Kumar, W.C. Kokke, M.F. Bean, A.J. Freyer, C.D. Brosse, S. Mai, A. Truneh, D.J. Faulkner, B. Carte, A.L. Breen, R.P. Hertzberg, R.K. Johnson, J.W. Westley, B.C.M. Potts, J. Org. Chem. 60, 1182 (1995)
B. Ramesh, C.M. Bhalgat, Eur. J. Med. Chem. 46, 1882 (2011)
H.M. Savanur, R.G. Kalkhambkar, G. Aridoss, K.K. Laali, Tetrahedron Lett. 57, 3029 (2016)
D.P. Narayanan, A. Gopalakrishnan, Z. Yaakob, S. Sugunan, B.N. Narayanan, Arab. J. Chem. https://doi.org/10.1016/j.arabjc.2017.04.011 (2017)
S. Zolfagharinia, E. Kolvari, N. Koukabi, Catal. Lett 147, 1551 (2017)
M. Sheykhan, A. Yahyazadeh, L. Ramezani, Mol. Catal. 435, 166 (2017)
E. Kolvari, N. Koukabi, O. Armandpour, Tetrahedron 70, 1383 (2014)
A. Phukan, S.J. Borah, P. Bordoloi, K. Sharma, B.J. Borah, P.P. Sarmah, D. Dutta, Adv. Powder Technol. 28, 1585 (2017)
K. Gong, H. Wang, S. Wang, X. Ren, Tetrahedron 71, 4830 (2015)
A. Khorshidi, K. Tabatabaeian, H. Azizi, M.A. Hashjin, E.A. Gilandeh, RSC Adv. 7, 17732 (2017)
P. Shen, M. Xu, D. Yin, S. Xie, C. Zhou, F. Li, Catal. Commun. 77, 18 (2016)
E. Kolvari, N. Koukabi, M.M. Hosseini, M. Vahidian, E. Ghobadi, RSC Adv. 6, 7419 (2016)
D. Bhuyan, M. Saikia, L. Saikia, Microporous Mesoporous Mater. 256, 39 (2018)
B.H. Rotstein, S. Zaretsky, V. Rai, A.K. Yudin, Chem. Rev. 114, 8323 (2014)
R.C. Cioc, E. Ruijter, R.V.A. Orru, Green Chem. 16, 2958 (2014)
R. Ramesh, A. Lalitha, RSC Adv. 5, 51188 (2015)
R. Ramesh, N. Nagasundaram, D. Meignanasundar, A. Lalitha, Res. Chem. Intermed. 43, 1767 (2017)
R. Ramesh, P. Kalisamy, J.G. Malecki, A. Lalitha, Synlett 29, 203 (2018)
R. Ramesh, G. Sankar, J.G. Malecki, A. Lalitha, J. Iran. Chem. Soc. 15, 1 (2018)
R. Ramesh, D. Meignanasundar, A. Lalitha, ChemistrySelect 2, 10210 (2017)
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H.J. Puschmann, J. Appl. Cryst. 42, 339 (2009)
G.M. Sheldrick, Acta Cryst A64, 112 (2008)
Acknowledgements
R. Ramesh (IF120729) gratefully thank the financial assistance from Department of Science and Technology (Grant No: DST/INSPIRE Fellowship/2012/690) New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramesh, R., Ramesh, S., Malecki, J.G. et al. Ultrasound aided solvent-free synergy: an improved synthetic approach to access 3,4-dihydropyrimidin-2(1H)-ones. J IRAN CHEM SOC 16, 1197–1205 (2019). https://doi.org/10.1007/s13738-019-01597-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01597-3