Abstract
A convenient and direct approach has been developed for the preparation of bis(indole) derivatives by one-pot four-component condensing of indole, aldehydes and active methylene compounds in the presence of 12-tungstophosphoric acid in aqueous media under silent and ultrasound methods. The remarkable advantages are the simplicity of the experimental procedures, short reaction times and high yields with the green aspects by avoiding toxic catalysts and solvents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bis(indole)-based compounds have received much attention as important building blocks for the synthesis of various biological active compounds [1–6]. Bis(indole) derivatives have been prepared via condensation reactions of Indole with various aldehydes or ketones in the presence of either protic or Lewis acids [7–9]. Ultrasound irradiation is a powerful technique in synthetic organic chemistry. Enhanced reaction rates, simple experimental procedure, and high yields are the notable features of the ultrasound approach as compared to established methods [10, 11]. Organic synthesis in aqueous media is gaining importance in view of the fact that the use of many toxic and volatile organic solvents contributes to pollution. There have been profound research activities in the development of organic reactions in aqueous media offering key advantages such as rate enhancement and insolubility of the final products, which facilitates their isolation by simple filtration [12–14]. Keggin-type heteropolyacids (HPAs) have been studied extensively for organic synthetic processes as acid or redox catalysts in homogeneous and heterogeneous media [15, 16]. HPAs catalyzed several organic transformations such as Diels–Alder reactions [17], oxidative dehydrogenation of alcohols and amines [18], olefin hydration [19], synthesis of dihydropyrimidinones [20], preparation of oximes [21] and synthesis of oxazolines, imidazolines and thiazolines [22]. The present work describe a simple method for the preparation of bis(indole) derivatives by one-pot four-component condensing of indole, aldehydes and active methylene compounds in the presence of 12-tungstophosphoric acid in aqueous media under silent and ultrasound irradiation methods (Scheme 1).
Results and discussion
In our initial study, the reaction of indole, benzaldehyde and malononitrile was considered as a model reaction to optimize the conditions. The reaction was first carried out in H2O in the absence of H3PW12O40 and at 95 °C. No reaction occurred under silent and ultrasound irradiation conditions (Table 1, entry 1). Similar reactions were then attempted in the presence of 1.3, 2.1, 2.7, 3.4 and 4.1 mol % of H3PW12O40. The results in Table 1 entries 2–6 show that the use of 3.4 mol% of H3PW12O40 in H2O at 95 °C is sufficient to push the reaction forward. Higher reaction loading of the catalyst had no significant influence on the reaction yield. To find the optimum reaction temperature, the reaction was carried out with 3.4 mol% of H3PW12O40 at room temperature, 60, and at 95 °C, which resulted in the isolation of product in a trace amount and 65 and 94 % yields (Table 1 entries 8, 7, and 5) respectively. In addition, EtOH, MeOH, AcOEt, and MeCN were also tested as the solvents. In these cases, 2-((di(1H Indol-3-yl)(phenyl)methyl)malononitrile was formed in lower yields (Table 1, entries 9–12). Thus, 3.4 mol % of H3PW12O40 in H2O at 95 °C was the optimal conditions. Ultrasound irradiation accelerated such reactions (Table 1, entry 13).

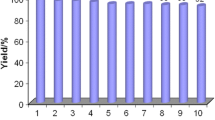
Under optimized conditions, bis(indole) derivatives were synthesized in high yields under silent and ultrasound irradiation conditions. The results are summarized in Table 2. It can be observed that the process tolerates both electron-donating and withdrawing substituent in the benzaldehyde. In all cases, the reactions proceeded efficiently at reflux under mild conditions to afford the corresponding products in high yields. All the products were characterized by 1H-, 13C-NMR and IR spectra and elemental analyses.

As expected, the reaction could be extended to other aldehydes under the optimized conditions. 2- and 3-pyridinecarboxaldehyde were also chosen to react with Indole and active methylene compounds. The reaction proceeded smoothly as expected in high yields. However, we wanted to obtain the expected bis(indole) derivatives but only indolyl derivatives were obtained. A possible reason for this is that oxidative dehydrogenation of 2-((indolyl)(pyridyl)methylene)malononitriles and 3-(indolyl)(pyridyl)acrylates in the presence of H3PW12O40 is less reactive than 2-((aryl)indolyl)methyl)malononitriles and 3-(aryl)(indolyl)acrylates respectively. 1H- and 13C-NMR spectra of the crude mixture clearly indicate that formation of the product lead to one stereoisomer. The results are summarized in Table 3. To explore the scope and limitations of this reaction further, we extended our studies to the reaction of pyrrole, aldehydes and active methylene compounds in the present of 12-tungstophosphoric acid as catalyst in aqueous media at 90 °C under silent conditions. The reaction proceeded smoothly and a black sticky mixture was achieved after 3 h. In accord with the literature, we think that pyrrole as an acid sensitive compound undergoes polymerization reactions without any Michael addition reactions occurring.

Experimental section
General
Melting points were determined by Büchi melting point B-540 B.V.CHI apparatus in open capillaries and are uncorrected. IR spectra were recorded as KBr pellets on a Bruker, Eqinox 55 spectrometer.1H- and 13C-NMR spectra were obtained in CDCl3 with Me4Si as the internal standard with a Bruker Avance 500 MHz spectrometer. Elemental analyses were carried out with a Costech ECS 4010 CHN analyzer. Analytical TLC was performed on pre-coated plastic sheets of silica gel G/UV-254 of 0.2 mm thickness.
Typical experimental procedure
Silent method
A mixture of Indole (0.12 g, 1 mmol), malononitrile (0.5 mmol, 0.03 g), benzaldehyde (0.5 mmol, 0.06 g), and catalyst (3.4 mol %) was refluxed at 95 °C in water (8 mL) for appropriate time. The completion of the reaction was monitored by TLC. After cooling the resulting precipitate was filtered, and recrystallized from ethanol to afford the pure product. The oily mixture was firstly extracted by CH2Cl2, and then the residue was purified by column chromatography eluting with chloroform/n-hexane, 9:1.
Ultrasound method
A mixture of indole (0.12 g, 1 mmol), malononitrile (0.5 mmol, 0.03 g), benzaldehyde (0.5 mmol, 0.06 g), and catalyst (3.4 mol %) in water (8 mL) was subjected to ultrasound irradiation at 95 °C for appropriate time. The completion of the reaction was monitored by TLC. After cooling the resulting precipitate was filtered, and recrystallized from ethanol to afford the pure product. The oily mixture was firstly extracted by CH2Cl2, and then the residue was purified by column chromatography eluting with chloroform/n-hexane, 9:1.
Spectroscopic deta
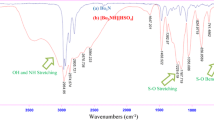
2-(Di(1H-indol-3-yl)(phenyl)methyl)malononitrile (Table 2, entry 1). IR (KBr, cm−1): 3409, 3050, 2923, 2160, 1606, 1475. 1H-NMR (500 MHz, CDCl3) δ: 5.94 (s, 1H, CH), 6.67 (s, 2H, CH), 7.07 (t, J = 6.4 Hz, 2H, ArH), 7.23 (t, J = 6.4 Hz, 2H, ArH), 7.27–7.35 (m, 7H, ArH), 7.45 (d, 2H, J = 6.9 Hz, ArH), 7.88 (s, 2H, NH). 13C-NMR (125 MHz, CDCl3) δ: 40.6, 77.7, 112.7, 111.4, 119.6, 120.1, 120.3, 122.3, 124.0, 126.5, 127.5, 128.6, 129.1, 137.1, 144.4; IR (KBr, cm−1): υ 3409, 3050, 2923, 2160, 1606, 1475. Anal. calcd. for C26H18N 4: C, 80.81; H, 4.69; N, 14.50 %. Found: C, 80.5; H, 4.4; N, 14.2 %.
2-(Di(1H-indol-3-yl)(4-nitrophenyl)methelyl)malononitrile (Table 2, entry 2). IR (KBr, cm−1): 3422, 3052, 2923, 1507, 1456, 1341. 1H-NMR (500 MHz, CDCl3) δ: 5.93 (s, 1H, CH), 6.64 (s, 2H, CH), 6.92 (t, J = 7.4 Hz, 2H, ArH), 7.09 (t, J = 7.4 Hz, 2H, ArH), 7.26 (d, J = 7.9 Hz, 2H, ArH), 7.34 (d, J = 8.1 Hz, 2H, ArH), 7.46 (d, J = 8.3 Hz, 2H, ArH), 8.06 (d, J = 8.7 Hz, 2H, ArH), 9.30 (s, 2H, NH). 13C-NMR (125 MHz, CDCl3) δ: 36.0, 48.9, 111.0, 113.0, 113.3, 119.8, 120.1, 122.0, 123.8, 124.0, 128.0, 130.0, 138.5, 148.0, 149.1; Anal. calcd. for C26H17N5O2: C, 72.38; H, 3.97; N, 16.23 %. Found: C, 72.1; H, 3.6; N, 16.4 %.
2-((2-Fluorophenyl)di(1H-indol-3-yl)methyl)malononitrile (Table 2, entry 3). IR (KBr, cm−1): 3392, 3056, 1580, 1438, 1219. 1H-NMR (500 MHz, CDCl3) δ: 6.28 (s, 1H, CH), 6.73 (s, 2H, CH), 7.09 (m, 3H, ArH), 7.13 (t, J = 9.1 Hz, 1H, ArH), 7.28 (m, 4H, ArH), 7.39 (d, J = 8.1 Hz, 2H, ArH), 7.46 (d, J = 7.9 Hz, 2H, ArH), 7.89 (s, 2H, NH). 13C-NMR (125 MHz, CDCl3) δ: 36.0, 42.1, 111.4, 112.2, 113.0, 116.0, 118.7, 119.7, 120.2, 122.4, 124.0, 127.3, 128.0, 129.0, 130.7, 137.1, 159.0. Anal. calcd. for C26H17N4F: C, 77.21; H, 4.24; N, 13.85 %. Found: C, 77.5; H, 4.5; N, 13.5 %.
2-((4-Fluorophenyl)di(1H-indol-3-yl)methyl)malononitrile (Table 2, entry 4). IR (KBr, cm−1): 3408, 3052, 2923, 1601, 1416, 217, 743. 1H-NMR (500 MHz, CDCl3) δ: 5.90 (s, 1H, CH), 6.33 (s, 2H, CH), 7.10 (m, 2H, ArH), 7.27–7.19 (m, 4H, ArH), 7.30 (m, 2H, ArH), 7.82 (d, 2H, ArH), 8.00 (d, 2H, Ar), 9.30 (s, 2H, NH). 13C-NMR (125 MHz, CDCl3) δ: 39.9, 48.0, 112.0, 112.8, 113.0, 116.0, 119.0, 120.7, 121.2, 123.8, 128.4, 130.0, 136.8, 138.0, 159.1. Anal. calcd. for C26H17N4F: C, 77.21; H, 4.24; N, 13.58 %. Found: C, 77.0; H, 4.6; N, 13.4 %.
2-((4-Chlorophenyl)di(1H-indol-3-yl)methyl)malononitrile (Table 2, entry 5). IR (KBr, cm−1): 3411, 2923, 1616, 1415, 744. 1H-NMR (500 MHz, CDCl3) δ: 5.89 (s, 1H, CH), 6.66 (s, 2H, CH), 6.93–7.04 (m, J = 8.1 Hz, 4H, ArH), 7.21 (d, J = 7.9 Hz, 2H, ArH,), 7.44 (d, J = 7.3 Hz, 2H, ArH), 7.80 (d, J = 8.1 Hz, 2H, ArH), 8.20 (d, 2H, ArH), 8.60 (s, 2H, NH). 13C-NMR (125 MHz, CDCl3) δ: 37.6, 50.0, 112.0, 113.0, 113.8, 118.5, 120.0, 121.5, 121.7, 125.5, 128.3, 130.0, 131.9, 138.0, 140.0. Anal. calcd. for C26H17N4Cl: C, 74.19; H, 4.07; N, 13.31 %. Found: C, 74.4; H, 4.3; N, 13.5 %.
2((3,4-Dimethoxyphenyl)di(1H-indol-3-yl)methyl)malononitrile (Table 2, entry 7). IR (KBr, cm−1): 3409, 3055, 2926, 2250, 1593, 1590. 1H-NMR (500 MHz, CDCl3) δ: 3.80 (s, 3 H, CH3), 3.89 (s, 3H, CH3), 5.88 (s, 1H, CH), 6.71 (s, 2H, CH), 6.81 (d, J = 8.2 Hz, 1H, ArH), 6.88 (dd, J 1 = 8.4 Hz, J 2 = 1.8 Hz, 1H, ArH), 6.67 (d, J = 1.8 Hz, 1H, ArH), 7.05 (t, J = 7.5 Hz, 2H, ArH), 7.21 (t, J = 7.5 Hz, 2H, ArH), 7.40 (d, J = 8.1 Hz, 2H, ArH), 7.45 (d, J = 7.9 Hz, 2H, ArH), 7.96 (s, 2H, NH). 13C-NMR (125 MHz, CDCl3) δ: 38.0, 50.0, 57.3, 57.4, 110.0, 112.0, 112.8, 115.0, 117.7, 119.7, 120.0, 122.0, 123.0, 123.7, 128.7, 133.0, 136.5, 148.0, 150.1. Anal. calcd. for C28H22O2N4: C, 75.32; H, 4.97; N, 12.55 %. Found: C, 75.5; H, 4.6; N, 12.2 %.
2-((4-Hydroxyphenyl)di(1H-indol-3-yl)methelyl)malononitrile (Table 2, entry 8). IR (KBr, cm−1): 3407, 3055, 2922, 1611, 1455. 1H-NMR (500 MHz, CDCl3) δ: 5.40 (s, 1H, CH), 5.30 (s, 1H, OH), 6.67 (s, 2H, NH), 6.8 (d, J = 8.2 Hz, 2H, ArH), 7.06 (m, 2H, ArH), 7.14 (d, J = 7.9 Hz, 2H, ArH), 7.60 (m, 2H, ArH), 7.90 (d, J = 8.2 Hz, 2H, ArH), 7.97 (d, J = 8.2 Hz, 2H, ArH), 8.30 (s, 2H, NH). 13C-NMR (125 MHz, CDCl3) δ: 39.0, 49.0, 111.0, 113.0, 116.0, 119.6, 121.1, 123.0, 128.2, 129.0, 129.3, 132.7, 136.5, 138.0, 155.5. Anal. calcd. for C26H18N4O: C, 77.59; H, 4.51; N, 13.92 %. Found: C, 77.6; H, 4.7; N, 13.6 %.
2-((1H-indol-3-yl)(pyridine-3-yl)methyl)malononitrile (Table 3, entry 1). IR (KBr, cm−1): 3407, 3075, 2902, 2258. 1H-NMR (500 MHz, CDCl3) δ: 4.8 (d, J = 6.9 Hz, 1H, CH), 4.99 (d, J = 5.0 Hz, 1H, CH), 6.00–7.90 (m, 7H, ArH), 8.42 (s, 1H, CH), 8.61 (s, 1H, CH), 10.37 (s, 1H, NH). 13C-NMR (125 MHz, CDCl3) δ: 29.5, 42.0, 110.9, 112.2, 112.9 116.1 118.6, 120.0, 122.7, 123.3, 124.0, 134.1, 136.1, 136.9, 150.0, 150.1. Anal. calcd. for C17H12N4: C, 74.89; H, 4.44; N, 20.58 %. Found: C, 74.5; H, 4.6; N, 20.5 %.
Ethyl 2-cyano-3- (1H-indol-3-yl)-3-(pyridine-3-yl)propanoate (Table 3, entry 5): IR (KBr, cm−1): 1260, 1732, 2252, 3411, 3040, 2980. 1H-NMR (500 MHz, CDCl3) δ: 1.06 (t, J = 7.1 Hz, 3H, CH3), 4.08 (q, J = 6.1 Hz, 2H, CH2), 4.34 (d, J = 6.2 Hz, 1H, CH), 5.01 (d, J = 6.2 Hz, 1H, CH), 6.80–7.90 (m, 7H, ArH), 8.45 (d, J = 3.9 Hz, 1H, CH), 8.63 (s, 1H, CH), 10.04 (s, 1H, NH). 13C-NMR (125 MHz, CDCl3) δ: 14.1, 38.0, 40.9, 63.4, 111.1, 112.8, 116.1, 118.6, 119.9, 122.1, 122.6, 123.0, 124.0, 133.2, 135.3, 136.9, 148.2, 149.9, 165.2; Anal. calcd. for C19H17N3O2: C, 71.46; H, 5.37; N 13.16 %. Found: C, 71.2; H, 5.7; N, 13.4 %.
Ethyl 2-cyano-3- (1H-indol-3-yl)-3-(pyridine-4-yl)propanoate (Table 3, entry 6). IR (KBr, cm−1): 3402, 2981, 2250, 1741, 1599, 1458. 1H-NMR (500 MHz, CDCl3). δ: 1.06 (t, J = 7.1 Hz, 3H, CH3), 4.08 (q, J = 6.1 Hz, 2H, CH2), 4.34 (d, J = 6.2 Hz, 1H, CH), 5.01 (d, J = 6.2 Hz, 1H, CH), 7.18 (m, 4H, ArH), 8.6 (d, J = 7.9 Hz, 2H, ArH), 7.28 (d, J = 8.2 Hz, 2H, ArH), 8.63 (s, 1H, CH), 10.04 (s, 1H, NH). 13C-NMR (125 MHz, CDCl3) δ: 14.1, 38.1, 40.09, 63.4, 112.0, 117.4, 120.7, 121.7, 122.6, 123.2, 124.5, 124.7, 137.1, 141.1, 150.0, 153.4, 164.5. Anal. calcd. for C19H17N3O2: C, 71.46; H, 5.37; N, 13.16 %. Found: C, 71.2; H, 5.1; N, 13.5 %.
References
T.R. Garbe, M. Kobayashi, N. Shimizu, N. Takesue, M. Ozawa, H.J. Yukawa, Nat Prod 63, 596 (2000)
B. Jiang, C.G. Yang, J. Wang, J Org Chem 66, 4865 (2001)
V.T. Kamble, K.R. Kadam, N.S. Joshi, D.B. Muley, Cat Commun 8, 498 (2007)
M. Karthik, C.J. Mageshk, P.T. Perumal, M. Palanichamy, B. Arabindoo, V. Murugesan, Appl Cat A 286, 137 (2005)
M. Karthik, A.K. Tripathi, N.M. Gupta, M. Palanichamy, V. Murugesan, Cat Commun 5, 371 (2004)
X. Ge, S. Yannai, G. Rennert, N. Gruener, F.A. Fares, Biochem Bioph Res Co 228, 153 (1996)
M. Badini, A. Melloni, S. Tommasi, A. Umani-Ronchi, Synlett 8, 1199 (2005)
M. Chakrabarty, R. Mukherjee, A. Mukherji, Heterocycles 68, 1659 (2006)
B.S. Liao, J.T. Chen, S.T. Liu, Synthesis 20, 3125 (2007)
T.J. Mason, Chem Soc Rev 26, 443 (1997)
J.T. Li, G.F. Chen, J.X. Wang, T.S. Li, Synth Commun 29, 965 (1999)
R. Breslow, Acc Chem Res 24, 159 (1991)
R. Breslow, Acc Chem Res 37, 471 (2004)
T.J. Mason, E.C.D. Meulenaer, In practical considerations for process optimisation, synthetic organic sonochemistry (Plenum Press, New York, 1998)
H. Firouzabadi, A.A. Jafari, J Iran Chem Soc 2, 85 (2005)
M.T. Pope, Heteropoly and isopoly oxometalates (Springer, Berlin, 1993)
G.J. Meuzelaar, L. Maat, R.A. Sheldon, I.V. Kozhevnikov, Cat Lett 45, 249 (1997)
R. Neumann, M. Levin, J Org Chem 56, 5707 (1991)
M. Misono, N. Nojiri, Appl Cat 64, 1 (1990)
R. Fazaeli, S. Tangestaninejad, H. Aliyan, M. Moghadam, Appl Catal A Gen 309, 44 (2006)
R. Fazaeli, S. Tangestaninejad, H. Aliyan, Cat Commun 8, 205 (2007)
I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad, V. Mirkhani, S.F. Hojati, Polyhedron 27, 750 (2008)
Acknowledgments
The authors thank the Research Council of Yazd University for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Amrollahi, M.A., Kheilkordi, Z. H3PW12O40-catalyzed one-pot synthesis of bis(indole) derivatives under silent and ultrasonic irradiation conditions in aqueous media. J IRAN CHEM SOC 13, 925–929 (2016). https://doi.org/10.1007/s13738-016-0808-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0808-z