Abstract
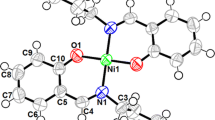
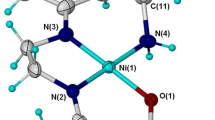
Three dihydroxylated acetophenone derivatives 2,6-(1a), 2,5-(2a), and 2,4-dihydroxyacetophenone (3a) were O-monoalkylated at moderate temperature (50 °C) using 3-bromopropyl-N-pyrrole. These monomers 6-(3′-N-pyrrolpropoxy)-2-hydroxyacetophenone (1b), 5-(3′-N-pyrrolpropoxy)-2-hydroxyacetophenone (2b), and 4-(3′-N-pyrrolpropoxy)-2-hydroxy acetophenone (3b) were isolated with acceptable yields (59–70 %). Their characterization was carried out with usual spectroscopic methods such as UV–vis, FTIR, NMR1H, 13C, Dept135, and elemental analysis. These pyrrolic compounds were deliberately chosen as electropolymerizable monomers to elaborate poly(pyrrole) films containing metallic centers useful as redox mediators covalently grafted on the surfaces of modified electrodes. Accordingly, we have initiated the synthesis of an original pyrrole-Ni(II)-Schiff base complex derived from 2,6-(1b) and 1,2-diaminoethane. This pyrrolic complex was electropolymerized onto glassy carbon (GC), platinum disk (Pt), and indium tin oxide (ITO) electrode surfaces. This electropolymerization was performed in acetonitrile via anodic oxidation of pyrrolic moieties by cyclic voltammetry. The efficiency of the electrochemical polymerization was investigated as a function of several parameters such as the nature of the electrode material, the number of voltammetric scans, and the scan rate dependence. The electrodeposited poly(pyrrole) films onto ITO surface was characterized by X-ray diffraction (XRD) and atomic force microscopy (AFM). This poly(pyrrole) matrix, containing metallic centers, was found to have good catalytic properties towards the reduction of iodobenzene and carbon dioxide CO2.
Graphical abstract












Similar content being viewed by others
References
K. Srinivasan, P. Michaud, J.K. Kochi, J. Am. Chem. Soc. 108, 2309 (1986)
C.P. Horwitz, S.E. Creager, R.W. Murray, Inorg. Chem. 29, 1006 (1990)
J.C. Moutet, A. Ourari, Electrochim. Acta. 42, 2525 (1997)
J. Qiao, I.D. Wang, I. Duan, Y. Li, D.Q. Zhang, Y. Qiu, Inorg. Chem. 43, 5096 (2004)
V. Ullrich, I. Roots, A. Hildebrandt, R.W. Eastbrook, A.H. Cooney (ed.) Microsomes and Drugs Oxidations (Pergamon Press, New York, 1975)
B. Halliwell, J.M.C. Gutterigde, Free radicals in biology and medicine. 2nd edn. (1988)
S. Cosnier, A. Le Pellec, B. Guidetti, I. Rico-Lattes, J. Electroanal. Chem. 449, 165 (1998)
N. Toshima, S. Hara, Prog. Polym. 20, 155 (1995)
A. Deronzier, J.C. Moutet, Coord. Chem. Rev. 147, 339 (1996)
O. Kocian, K. W. Chiu, R. Demeure, B. Gallez, C. J. Jones, J. R. Thornback, J. Chem. Soc. Perkin Trans, 527 (1994)
A. Ourari, K. Ouari, W. Moumeni, L. Sibous, Transit. Met. Chem. 31, 169 (2006)
A. Ourari, K. Ouari, G. Bouet, A.M. Khan, J. Coord. Chem. 61, 3846 (2008)
A. Ourari, L. Baameur, G. Bouet, A.M. Khan, Electrochem. Commun. 10, 1736 (2008)
G. Cauquis, S. Cosnier, A. Deronzier, B. Galland, D. Limosin, J. Moutet, J. Bizot, D. Deprez, J.P. Pullicani, J. Electroanal. Chem. 352, 181 (1993)
F. Bedioui, E. Labbe, S. Gutierrez-Granados, J. Devynck, J. Electroanal. Chem, 267 (1991)
J. Losada, I.del.Peso, L. Beyer, J. Hartung, V. Fernandez, M. Mobius. J. Electroanal. Chem. 398, 89 (1995)
G.N. Vyas, N.M. Shah, Org. Synth. 4, 836 (1963)
B. De Binod, B. Lohray, Braj and K. Dahl Pradeep. Tetrahedron Lett. 34, 2371 (1993)
P. Guo, K.Y. Wong, J. Electrochem. Commun. 1, 559 (1999)
S. Shahrokhian, A. Souri, H. Khajehsharifi, J. Electroanal. Chem. 565, 95 (2004)
L.J. Boucher, J. Inorg. Nucl. Chem. 36, 531 (1974)
J.P. Costes, M.I. Fernandez-Gracia, Inorg. Chim. Acta. 237, 57 (1995)
E. Kiatkowski, G. Romanowski, W. Nowicki, M. Kiatkowski, K. Suwinska, Polyhdron. 22, 1009 (2003)
E.G. Samsel, K. Srinivasan, J.K. Kochi, J. Am. Chem. Soc. 107, 7606 (1985)
Y. Zidane, A. Ourari, T. Bataille, P. Hapiot, D. Hauchard, J. Electroanal. Chem. 641, 64 (2010)
J. Losada, I. Del Peso, L. Beyer, J. Electroanal. Chem. 447, 147 (1998)
J. Losada, I. Del Peso, L. Beyer, Inorg. Chim. Acta 321, 107 (2001)
M.G. Gichinga, S. Stregler, Tetrahedron 65, 4917 (2009)
G. Cafeo, F.H. Kohnke, L. Valenti, Tetrahedron Lett. 50, 4138 (2009)
A. Ourari, D. Aggoun, S. Bouacida, Acta. Cryst. E68, 1083 (2012)
H. Carpio, E. Galeazzi, A. Greenhouse, A. Guzman, E. Velarde, Y. Antonio, F. Franco, A. Leon, V. Perez, R. Salas, D. Valdès, J. Ackrell, D. Cho, P. Gallegra, O. Halpeth, R. Koehler, M.L. Maddox, J.M. Muchowski, A. Prince, D. Tegg, T.C. Thurber, A.R. Vanhorn, D. Wren, Can. J. Chem. 60, 2295 (1982)
R. Aurkie, M.G. Rosair, R. Kadam, S. Mitra, Polyhedron 28, 796 (2009)
Y.M. Issa, M.M. Omar, H.A. Abdel-Fatah, A.A. Soliman, Egypt. J. Chem. 38, 249 (1995)
A.A. Soliman, Spectrochimica Acta Part A. 53, 509 (1997)
M.M. Abd-Elzaher, J. Chin. Chem. Soc. 48, 153 (2001)
R.M. Silverstein, G.C. Bassler, T.C. Morrill, Spectrometric identification of organic compounds, 4th edit (Wiley, New York, 1981)
E.B. Seena, M.R. Prathapachandra Kurup, Spectrochim. Acta A. 69, 726 (2008)
H. Temel, S. Ilhan, M. Sekerci, Synt. React. Inorg. Met. Org. Chem. 32(9), 1625 (2002)
A.B.P. Lever, Inorganic electronic spectroscopy, 2nd edn. (Elsevier, London, 1992)
S. Djebar-Said, O. BenaliBaitich, J.P. Deloume, J. Mol. Struct. 569, 121 (2001)
C. Rimington, Biochem. J. 75, 620 (1960)
K. Ouari, A. Ourari, J. Weiss, J. Chem. Crystallogr. 40, 831 (2010)
E. Cristopher, G. Dahm, D. Peters, Anal. Chem. 66, 3117 (1994)
A.R. Silva, C. Freire, B. de Castro, M.M.A. Freitas, J.L. Figueiredo, Microporous Mesoporous Mater. 46, 211 (2001)
A. Deronzier, J.C. Moutet, Acc. Chem. Res. 22, 249 (1989)
M.M. Ayad, J. Mater. Sci. 44, 6392 (2009)
D. Hodko, M. Gamboa-Aldeco, O.J. Murphy, J. Solid State Electrochem. 13, 1077 (2009)
G. Costa, A. Puxeddu, E. Reisenhofer, J. Chem. Soc. Dalton. Trans. 2034 (1973)
D.-L. Zhou, H. Carrero, J.F. Rusling, Langmuir 12, 3067 (1996)
A.A. Isse, A. Gennaro, E. Vianello, J. Electroanal. Chem. 444, 241 (1998)
S.A. Kaufman, T. Phanijphand, A.J. Fry, Tetrahedron Lett. 37, 8105 (1996)
C. Ji, S.E. Day, W.C. Silvers, J. Electroanal. Chem. 622, 15 (2008)
A. Behr, Carbon Dioxide Activation by Metal Complexes (VCH, Weinheim, 1988)
J.A. BrownBourzutschky, N. Horns, A.T. Bell, J. Catal. 124, 73 (1998)
C.P. Horwitz, S.E. Creager, R.W. Murray, Inorg. Chem. 29, 1006 (1990)
A. Jarzȩ bińska, P. Rowiński, I. Zawisza, R. Bilewicz, L. Siegfried, T. Kaden, Analytica. Chimica. Acta. 396, 1 (1999)
A. Murata, Y. Hori, Bull. Chem. Sot. Jpn. 64, 123 (1991)
Y. Hori, K. Kikuchi and S. Suzuki, Chem. Lett, 1695 (1985)
C. Amatore, J.-M. SavBant, J. Am. Chem. Sot. 103, 5021 (1981)
S. Ikeda, T. Takagi, K. Ito, Bull. Chem. Sot. Jpn. 60, 2517 (1987)
J.P. Collin, J.P. Sauvage, Coord. Chem. Rev. 93, 245 (1989)
Acknowledgments
The authors thank the Algerian Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et la Direction Générale de la Recherche for financial support and would also like to thank Professors Olivier BURIEZ and Christian AMATORE for helpful discussions, Ecole Normale Supérieure Département de Chimie, UMR CNRS-ENS-UPMC 8640, 24 rue Lhomond, 75231 Paris Cedex 5. France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ourari, A., Aggoun, D. Synthesis and spectral analysis of N-substituted pyrrole salicylaldehyde derivatives-electropolymerization of a new nickel(II)-Schiff base complex derived from 6-[3′-N-pyrrolpropoxy]-2-hydroxyacetophenone and 1,2-diaminoethane. J IRAN CHEM SOC 12, 1893–1904 (2015). https://doi.org/10.1007/s13738-015-0664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0664-2