Abstract
A 62-year-old man with type 2 diabetes was admitted because of a decrease in estimated glomerular filtration rate from 72 to 17.5 mL/min/1.73 m2 in 10 years and development of widespread bullous skin lesions. His hemoglobin A1c level had been maintained at 6.0–7.0% for 10 years with a dipeptidyl peptidase (DPP)-4 inhibitor. Skin biopsy showed typical bullous pemphigoid, and kidney biopsy showed tubulointerstitial nephritis with eosinophilic infiltration and glomerular endothelial cell proliferation. After discontinuing the DPP-4 inhibitor, skin lesions improved, and renal decline slowed. This case indicates that DPP-4 inhibitors can cause not only skin lesions but also renal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dipeptidyl peptidase-4 (DPP-4) inhibitors are widely used for the treatment of type 2 diabetes (T2D) [1]. Since Skandalis et al. reported on the relationship between DPP-4 inhibitors and bullous pemphigoid (BP) in 2012 [2], many case reports of BP associated with DPP-4 inhibitors have been published in different countries [3]. In addition to their antihyperglycemic effects, DPP-4 inhibitors promote post-injury regeneration of endothelium and myocardium and may inhibit the development of atherosclerosis [4].
We experienced a case of BP during DPP-4 inhibitor use. In addition, the patient also developed nephropathy, which was characterized by progressive renal function decline. After discontinuation of the DPP-4 inhibitor, skin findings improved and the progression of renal failure slowed. We conclude that although DPP-4 inhibitors have an excellent effect in controlling blood glucose in patients with T2D, they may cause not only skin lesions but also renal complications.
Case report
A 62-year-old Japanese man was admitted to our hospital for evaluation of renal dysfunction and skin lesions. At age 40 years, he was diagnosed with T2D. He was treated by a diabetes specialist with diet and an alpha-glucosidase inhibitor and had very good glycemic management. At age 51 years, the patient started treatment with the DPP-4 inhibitor sitagliptin. At that time, creatinine was 0.87 mg/dL, the estimated glomerular filtration rate (eGFR) was 72 mL/min/1.73 m2, and urine protein was 2+ in the qualitative test. In the subsequent 10 years, the hemoglobin A1c (HbA1c) level was maintained at 6.0–7.0%.
At age 61 years, the patient was switched from sitagliptin to vildagliptin, and one month later, widespread bullous eczema appeared. Vildagliptin was discontinued. The patient’s skin lesions were treated with conservative dermatological treatments, such as steroid ointments, and slightly improved. Four months after vildagliptin was discontinued, sitagliptin was started again. One month later, the skin rash worsened, and during the course of treatment, renal dysfunction slowly progressed. Consequently, the patient was admitted to our hospital.
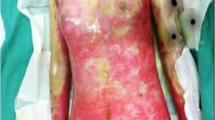
On admission, the patient was 172 cm tall and weighed 80 kg. His blood pressure was 151/70 mm Hg; pulse rate, 70 beats per minute; and body temperature, 36.1 °C. Bullous lesions were present all over his skin (Fig. 1a). The bilateral lower legs were edematous. No abnormalities of the heart, lungs, or abdomen were found.
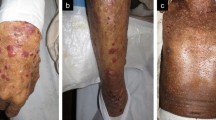
Skin lesions. a Numerous bullous lesions on the lower legs. b Skin biopsy with subepidermal rip (blisters; arrow); hematoxylin–eosin stain (original magnification × 40). c Inflammatory cell infiltrate, mainly eosinophils, in the dermis; hematoxylin–eosin stain (original magnification × 200). d Linear deposition of immunoglobulin G (arrow) at the dermo-epidermal junction in immunofluorescence analysis
Laboratory findings were as follows (Table 1): leucocytes, 9100/μL; hemoglobin, 9.9 g/dL; thrombocytes, 211,000/μL; creatinine, 3.05 mg/dL; eGFR, 17.5 mL/min/1.73 m2; glucose, 222 mg/dL; HbA1c, 5.9%; triglyceride, 125 mg/dL; total cholesterol, 177 mg/dL; total complement activity (assessed as CH50), 53 U/mL (normal value, > 30 U/mL); complement 3, 74 mg/dL (reference range 86–160 mg/dL); complement 4, 26 mg/dL (reference range 17–45 mg/dL); immunoglobulin G (IgG), 1467 mg/dL (reference range 861–1747 mg/dL); rheumatoid factor, 56 U/mL (reference range < 10.0 U/mL); cyclic citrullinated peptide antibodies, 742 U/mL (reference range: < 4.5 U/mL); and anti-bullous pemphigoid 180 antibody, 5.9 U/mL (reference range: < 9 U/mL). The various other autoantibody tests, including anti-dsDNA antibody, anti-Sm antibody, anti-SS-A/Ro antibody, anti-RNP antibody, anti-desmoglein 1 antibody, and anti-desmoglein 3 antibody, were all negative. Urinary protein excretion was 4.6 g/day, and the sediment contained 1–4 erythrocytes per high-power field. The level of urinary N-acetyl-β-d-glucosaminidase was 17.3 IU/day and that of urinary β2-microglobulin, 0.7 mg/day.
Chest X-ray showed an increased cardiothoracic ratio and left-sided pleural effusion. Computed tomography showed bilateral atrophic kidneys, with a long diameter of 84 mm.
Kidney biopsy
Light microscopy examination of a biopsy sample containing 46 glomeruli revealed global sclerosis of 12 glomeruli. Marked endothelial cell proliferation, glomerular basement membrane duplication, and lobular structure were characteristic findings in the preserved glomeruli (Fig. 2a). Nodular sclerosis was not seen. Arteriolar hyalinosis was mild (Fig. 2b), but interlobular arteries showed moderate fibroelastosis. Polar vasculosis was observed (Fig. 2a). Interstitial fibrosis and tubular atrophy with focal moderate infiltration of inflammatory cells, including lymphocytes, plasma cells, and massive eosinophils (Fig. 2h), occupied approximately 70% of the cortical area (Fig. 2g). Immunofluorescence microscopy showed no staining for IgG (Fig. 2c), IgA, IgM, C3, C4, or C1q. Electron microscopy of preserved glomeruli showed endothelial proliferation, subendothelial space enlargement, and partial foot process effacement, but electron-dense deposits were not detected. The glomerular basement membrane was thickened (600–800 nm) (Fig. 2f). Immunohistological analysis also showed CD34-positive endothelial cell proliferation (Fig. 2d). These findings reconfirmed the diagnosis of class 2A diabetic nephropathy according to Tervaert’s pathologic classification [5]. However, the biopsy sample was characterized also by tubulointerstitial nephritis with eosinophilic infiltration and by glomerular microangiopathy with endothelial cell proliferation, findings that are not typical for diabetic nephropathy.
Kidney biopsy. a Marked endothelial cell proliferation (arrow head), glomerular basement membrane duplication (black arrow), and lobular structure, with polar vasculosis (white arrow); periodic acid methenamine silver stain (original magnification × 400). b Mild arteriolar hyalinosis (arrow); periodic acid methenamine silver stain (original magnification × 400). c No staining for immunoglobulin G in immunofluorescence microscopy. d CD34-positive endothelial cell proliferation (arrow) in immunohistological analysis. e Scattered CD68-positive cells in the glomerulus. f Preserved glomeruli with endothelial proliferation (arrow) and subendothelial space enlargement (*) in electron microscopy. g Interstitial fibrosis and inflammatory cell infiltration occupying approximately 70% of the cortical area; Masson trichrome stain (original magnification × 40). h Eosinophil infiltration (arrow); hematoxylin–eosin stain (original magnification × 200)
Clinical course
Sitagliptin was immediately discontinued because it was considered to be a potential cause of the skin manifestations and nephropathy. Post-hospitalization nutritional dietary treatment with restriction of salt intake to 6 g/day and water intake to 1 L/day and continuous use of angiotensin II receptor antagonists and calcium channel blockers led to a body weight loss of 4 kg and a reduction in urinary protein to approximately 1.5 g/day, indicating slowing of the progression of renal failure (Fig. 3). The skin lesions also subsided with continued conservative dermatological treatment with steroid ointments. Immunosuppressive drugs, including steroids, were not administered, as the discontinuation of sitagliptin and the conservative management described above had reduced not only the skin lesions but also the exacerbation of renal function.
Discussion
Although the patient had good glycemic control for the 10 years after starting treatment with a DPP-4 inhibitor, renal dysfunction progressed over time, and the appearance of skin lesions led us to speculate that the skin and renal lesions might be related to DPP-4 inhibitor treatment. Characteristic BP lesions have been reported as skin lesions caused by DPP-4 inhibitors [2, 3]. However, to our knowledge, renal lesions associated with DPP-4 inhibitors have not been reported to date. Of relevance is that the characteristics of DPP-4 inhibitor-induced renal injury seen in this patient differ from those seen in typical T2D nephropathy. Therefore, when reviewing this case, the reports by Mise et al. on T2D nephropathy are useful [6, 7]. According to these reports, the mean creatinine level of patients with T2D diagnosed with class 2A diabetic nephropathy is 1.2 mg/dL, mean eGFR is 56.9 mL/min/1.73 m2, and mean urinary protein is 1.99 g/day. A comparison of these values with those in the present patient shows that the patient’s renal function was clearly impaired and his urinary protein level was higher than that of patients with diabetic nephropathy class 2A.
The present case was also characterized by excessive tubular damage, mainly tubulointerstitial nephritis with eosinophilic infiltration. These characteristics were presumably related to a drug-induced allergic reaction. The glomerular lesions with endothelial cell proliferation were different from typical T2D nephropathy. Brenner et al. reported that DPP-4 inhibitors may promote regeneration of endothelium and myocardium after injury, thereby inhibiting the onset of atherosclerosis, i.e., they suggested that the proliferation of endothelial cells may protect against atherosclerosis [4]. Many other reports also support this idea [8]. This concept appears to apply to the present case because the hyalinization of the arterioles was mild considering that the patient had a long history of T2D and DPP-4 inhibitor was presumably protective against the development of arteriolar lesions. On the other hand, DPP-4 inhibitor may induce endothelial cell proliferation in the glomerulus, which may lead to pathological conditions other than atherosclerosis.
The DPP-4 inhibitor-induced glomerular lesions we reported are similar to the glomerular lesions reported by Eremina et al. as VEGF inhibitor-induced thrombotic microangiopathy [9]. A similar drug-induced mechanism seems to be involved.
Bullous pemphigoid developed after switching from sitagliptin to vildagliptin in this patient. This is in accordance with Arai et al.’s report. Who reported that vildagliptin is actually more likely to cause bullous pemphigoid among several DPP4 derivatives. However, the fact that bullous pemphigoid, which once improved after vildagliptin was discontinued, reappeared on resumption of sitagliptin may be a common feature of DPP4 derivatives [10].
The following discussion is useful because DPP-4 inhibitors have been associated not only with bullous pemphigoid but also with specific nephropathy. The functional role of DPP-4 inhibitors in organ homeostasis or pathology can be divided into two: enzyme-dependent or enzyme-independent mechanisms. In an enzyme-dependent role, the accumulation of CXCL12, a substrate of DPP-4, may be involved not only in the pathogenesis of pemphigoid but also in renal disease. The enzyme-independent role of DPP-4 inhibitors may be implicated in the endothelial proliferation observed in kidney biopsies. It has been suggested that DPP-4 inhibitors have potential effects on endothelial integrin and VEGF signaling pathways. DPP-4 inhibitor has shown to block DPP-4-integrin interaction and then induce VEGF receptors alterations. Glomerular endothelial proliferation was seen when VEGF signaling was too much stimulated. This altered level of integrins and VEGF signaling are also seen in bullous pemphigoid [11].
In conclusion, we experienced a patient with BP and renal impairment after DPP-4 inhibitor administration. After discontinuation of the drug, the skin lesions resolved and the renal decline slowed. The cause of the renal impairment was tubulointerstitial nephritis with eosinophilic infiltration and glomerular microangiopathy with endothelial cell proliferation.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- BP:
-
Bullous pemphigoid
- DN:
-
Diabetic nephropathy
- DPP-4:
-
Dipeptidyl peptidase-4
- T2D:
-
Type 2 diabetes
References
Remm F, Franz W, Brenner C. Gliptins and their target dipeptidyl peptidase 4: implications for the treatment of vascular disease. Eur Heart J Cardiovasc Pharmacother. 2015;2:185–93.
Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249–53.
Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid. Front Immunol. 2019;10:1238.
Brenner C, Franz WM, Kühlenthal S, Kuschnerus K, Remm F, Gross L. DPP-4 inhibition ameliorates atherosclerosis by priming monocytes into M2 macrophages. Int J Cardiol. 2015;199:163–9.
Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noël LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA, Renal Pathology Society. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–63.
Mise K, Hoshino J, Ubara Y, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Sawa N, Fujii T, Ohashi K, Hara S, Takaichi K. Renal prognosis a long time after renal biopsy on patients with diabetic nephropathy. Nephrol Dial Transpl. 2014;29(1):109–18.
Mise K, Yamaguchi Y, Hoshino J, Ueno T, Sekine A, Sumida K, Yamanouchi M, Hayami N, Suwabe T, Hiramatsu R, Hasegawa E, Sawa N, Fujii T, Hara S, Sugiyama H, Makino H, Wada J, Ohashi K, Takaichi K, Ubara Y. Paratubular basement membrane insudative lesions predict renal prognosis in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. PLoS ONE. 2017;12(8):e0183190.
Yang CJ, Fan ZX, Yang J, Yang J. DPP-4 inhibitors: a potential promising therapeutic target in prevention of atherosclerosis. Int J Cardiol. 2016;202:797–8.
Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–36.
Arai M, Shirakawa J, Konishi H, Sagawa N, Terauchi Y. Bullous pemphigoid and dipeptidyl peptidase 4 inhibitors: a disproportionality analysis based on the Japanese adverse drug event report database. Diabetes Care. 2018;41(9):e130–2.
Shi S, Srivastava SP, Kanasaki M, He J, Kitada M, Nagai T, Nitta K, Takagi S, Kanasaki K, Koya D. Interactions of DPP-4 and integrin β1 influences endothelial-to-mesenchymal transition. Kidney Int. 2015;88(3):479–89.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests and no conflicts of interest.
Ethics approval
The present report was produced in conformity with the Declaration of Helsinki, and the patient gave his written consent for this case report to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Suenaga, A., Sawa, N., Oba, Y. et al. A case of bullous pemphigoid and renal disease after dipeptidyl peptidase 4 inhibitor administration. CEN Case Rep (2023). https://doi.org/10.1007/s13730-023-00835-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13730-023-00835-1