Abstract
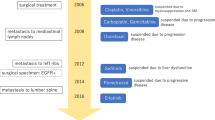
Bevacizumab is a monoclonal antibody against vascular endothelial growth factor (VEGF) that is used to treat patients with various cancers. However, it is known to be associated with adverse events, such as hypertension and proteinuria. The histology of bevacizumab-induced nephropathy is known as thrombotic microangiopathy or minimal change nephrotic syndrome. Recently, however, the terms “bevacizumab-associated glomerular microangiopathy” and “anti-VEGF therapy-induced glomerular microangiopathy” have been proposed. We present a case of a 68-year-old woman who was administered postoperative chemotherapy (carboplatin, paclitaxel, and bevacizumab) for stage IV ovarian cancer. Proteinuria and hypertension appeared after three courses; however, six courses were completed. Then, gemcitabine and carboplatin were administered for recurrence of her cancer. She was diagnosed with nephrotic syndrome after eight courses. Renal biopsy showed accumulation of periodic acid-Schiff (PAS)-positive substances in the capillary walls and para-mesangial areas. Double contouring of basement membranes was also observed. Immunofluorescence microscopy revealed positive staining for IgG, IgA, IgM, C3, C4, and C1q. Immunosuppressive therapy was administered, but was ineffective. Further examination by electron microscopy and immunostaining led to a diagnosis of bevacizumab-associated glomerular microangiopathy.



Similar content being viewed by others
References
Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21(8):1381–9. https://doi.org/10.1681/asn.2010020167.
Izzedine H. Anti-vegf cancer therapy in nephrology practice. Int J Nephrol. 2014. https://doi.org/10.1155/2014/143426.
Frangié C, Lefaucheur C, Medioni J, Jacquot C, Hill GS, Nochy D. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. 2007. https://oncology.thelancet.comvol.
Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–36. https://doi.org/10.1056/nejmoa0707330.
Hanna RM, Lopez E, Wilson J, Barathan S, Cohen AH. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J. 2016;9(2):239–44. https://doi.org/10.1093/ckj/sfv139.
Pfister F, Amann K, Daniel C, Klewer M, Büttner A, Büttner-Herold M. Characteristic morphological changes in anti-VEGF therapy-induced glomerular microangiopathy. Histopathology. 2018;73(6):990–1001. https://doi.org/10.1111/his.13716.
Person F, Rinschen MM, Brix SR, et al. Bevacizumab-associated glomerular microangiopathy. Mod Pathol. 2019;32(5):684–700. https://doi.org/10.1038/s41379-018-0186-4.
Roncone D. Proteinuria in a patient receiving anti-VEGF therapy for metastatic renal cell carcinoma. Nat Clin Pr Nephrol. 2007;3:287.
Tomita M, Ochiai M, Shu S, et al. A case of thrombotic microangiopathy with glomerular subendothelial IgA deposition due to bevacizumab. Jpn J Nephrol. 2014;56(5):612–7.
Yahata M, Nakaya I, Sakuma T, Sato H, Aoki S, Soma J. Immunoglobulin A nephropathy with massive paramesangial deposits caused by anti-vascular endothelial growth factor therapy for metastatic rectal cancer: a case report and review of the literature. BMC Res Notes. 2013. https://doi.org/10.1186/1756-0500-6-450.
Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF—a system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol - Ren Physiol. 2006;291(2):422–8. https://doi.org/10.1152/ajprenal.00448.2005.
Sugimoto H, Hamanog Y, Charytan D, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278(15):12605–8. https://doi.org/10.1074/jbc.C300012200.
Hara S, Tsuji T, Fukasawa Y, et al. Clinicopathological characteristics of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. Virchows Arch. 2019. https://doi.org/10.1007/s00428-019-02558-0.
Zhao N, Xu Q, Wang M, Fei X, Pan Y, Chen X, Ma S. Mechanism of kidney injury caused by bevacizumab in rats. Int J Clin Exp Pathol. 2004;7:8675–83.
Hoxha E, Beck LH, Wiech T, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7a-specific antibodies in membranous nephropathy. J Am Soc Nephrol. 2017;28(2):520–31. https://doi.org/10.1681/ASN.2016010050.
Acknowledgements
We thank Dr. Yoshihiko Ueda (emeritus professor of Dokkyo Medical University) and Dr. Kazushi Konma (Sakai City Medical Center) for useful advice. We also thank Mr. Takuya Okamura (Dokkyo Medical University Saitama Medical Center) for excellent technical assistance.
Funding
There were no specific funding sources relevant to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient in the case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Morimoto, M., Arai, T., Matsuura, M. et al. Bevacizumab-associated glomerular microangiopathy that occurred after postoperative chemotherapy for ovarian cancer. CEN Case Rep 10, 6–11 (2021). https://doi.org/10.1007/s13730-020-00504-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-020-00504-7