Abstract
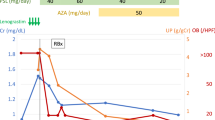
Ustekinumab (UST), an interleukin (IL)-12/IL-23-blocking monoclonal antibody, is a novel therapeutic option for Crohn’s disease (CD). We describe a 24-year-old man with CD who showed an abrupt decline in renal function after administration of UST. Twenty-nine months previously, the patient was diagnosed with CD, and abnormal urinalysis findings in health checkup were coincidentally found at that time. Three months previously, treatment for CD was switched from infliximab to UST because of therapy-resistant severe diarrhea and bloody stools. A single dose of UST (260 mg) was initially intravenously administered, followed by single subcutaneous administration (90 mg) 2 months later. Thereafter, the patient exhibited rapid renal dysfunction with significant urinary abnormalities, although his gastrointestinal symptoms had completely disappeared. He was admitted to our hospital for further examination and treatment. Renal pathologic findings were compatible with crescentic glomerulonephritis consisting of almost fibro-cellular crescents. Immunofluorescent study showed IgA and C3 deposition in the glomerular mesangial area and IgA subclass staining revealed predominant IgA1 with concomitant mild IgA2 deposition. Furthermore, galactose-deficient IgA1 (Gd-IgA1) was also positive in the mesangial area. In addition, serum-Gd-IgA1 level was moderately increased. UST treatment was stopped and he responded to intensive steroid therapy with a parallel reduction of serum creatinine and Gd-IgA1 levels without flare of gastrointestinal symptoms. To our knowledge, this is the first case of immunoglobulin A nephropathy (IgAN) in patient with CD that might be aggravated by UST treatment. We presume that inhibition of IL-12/23 signaling with UST may cause to form crescentic IgAN by enhancing Gd-IgA1 production.



Similar content being viewed by others
References
Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16(3):185–96.
Benson JM, Sachs CW, Treacy G, Zhou H, Pendley CE, Brodmerkel CM, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. 2011;29(7):615–24.
Battat R, Kopylov U, Bessissow T, Bitton A, Cohen A, Jain A, et al. Association Between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2017;15(9):1427–34 (e2).
Coppo R. Clinical and histological risk factors for progression of IgA nephropathy: an update in children, young and adult patients. J Nephrol. 2017;30(3):339–46.
Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118(2):629–39.
Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. 2014;124(6):2325–32.
Suzuki H, Yasutake J, Makita Y, Tanbo Y, Yamasaki K, Sofue T, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93(3):700–5.
Yasutake J, Suzuki Y, Suzuki H, Hiura N, Yanagawa H, Makita Y, et al. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant. 2015;30(8):1315–21.
Wada Y, Matsumoto K, Suzuki T, Saito T, Kanazawa N, Tachibana S, et al. Clinical significance of serum and mesangial galactose-deficient IgA1 in patients with IgA nephropathy. PLoS One. 2018;13(11):e0206865.
Suzuki H. Biomarkers for IgA nephropathy on the basis of multi-hit pathogenesis. Clin Exp Nephrol. 2019;23(1):26–31.
Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135(4):1130–41.
Sandborn WJ, Rutgeerts P, Gasink C, Jacobstein D, Zou B, Johanns J, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther. 2018;48(1):65–77.
World MJ, Stevens PE, Ashton MA, Rainford DJ. Mesalazine-associated interstitial nephritis. Nephrol Dial Transplant. 1996;11(4):614–21.
Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9(2):265–70.
Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, et al. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest. 2004;113(6):826–35.
Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347(10):738–48.
Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63(3):1164–77.
Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol. 2013;24(12):2118–255.
Marsal J, Agace WW. Targeting T-cell migration in inflammatory bowel disease. J Intern Med. 2012;272(5):411–29.
Ebrahimi Daryani N, Saghazadeh A, Moossavi S, Sadr M, Shahkarami S, Soltani S, et al. Interleukin-4 and interleukin-10 gene polymorphisms in patients with inflammatory bowel disease. Immunol Invest. 2017;46(7):714–29.
Ruszkowski J, Lisowska KA, Pindel M, Heleniak Z, Debska-Slizien A, Witkowski JM. T cells in IgA nephropathy: role in pathogenesis, clinical significance and potential therapeutic target. Clin Exp Nephrol. 2019;23(3):291–303.
Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem. 2014;289(8):5330–9.
Acknowledgements
We greatly appreciate the excellent technical assistance provided by Ms. Tomoko Suzuki.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Wada received Speakers Bureau from Mitsubishi Tanabe Pharma Corporation and T. Shibata has received research funding from Mitsubishi Tanabe Pharma Corporation, although the funder played no role in the present case of the manuscript.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient described in the present case.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kanazawa, N., Wada, Y., Akiyama, M. et al. Crescentic IgA nephropathy after administration of human monoclonal interleukin-12/23p40 antibody in a patient with Crohn’s disease: a case report. CEN Case Rep 9, 204–209 (2020). https://doi.org/10.1007/s13730-020-00457-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-020-00457-x