Abstract
This work was conducted with the objective of improving the physicochemical properties of flaxseed gum with added agar. Three formulations of flaxseed gum/agar (FG/AG) blends (75/25, 50/50 and 25/75) were studied in the presence of glutaraldehyde as crosslinking agent and a glycerol plasticizing agent. Cellulose nanocrystals (CNCs) were used as reinforcing agents at different levels (2%, 4% and 8% w/w) to prepare nanocomposite films by casting polymeric blends of flax seed and agar with both additives. The interaction with water (vapor permeability and solubility), scanning electron microscopy and tensile tests were performed for films, blends and nanocomposites. The flaxseed gum, agar and additives showed good homogeneity, as demonstrated by scanning electron microscopy. All the formulations presented tenacity increase between 585 and 811%, and lower values of solubility relative to the control film (FGcontrol). The best formulation of nanocomposites was 50/50–4% CNCs, that presented higher modulus of elasticity and increase in tenacity of 130% relative to the 50/50 blend and 1588% relative to the control film. The results showed that AG and CNC can modulate the properties of FG, opening numerous opportunities for investigation. Due to the significant improvement in the mechanical properties, FG/AG blends and nanocomposites have excellent potential in applications as sustainable packaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioplastics are designed as useful alternatives for conventional plastics. Petroleum-based materials could potentially be replaced by renewable and biodegradable materials [1]. The growing interest in the development of bioplastics brings opportunities to use polysaccharides, such as flaxseed gum [2] and agar [3].
Flaxseed gum (“gel-like” purified or in natura) has many potential applications in the food, cosmetic and pharmaceutical industries because of its nutritional and physicochemical properties [4]. It consists of a major neutral fraction composed of L-arabinose, D-xylose and D-galactose, and a minor acidic fraction composed of L-rhamnose, L-fucose, L-galactose and D-galacturonic acid [5, 6].
Another polymeric matrix which is important in this study is the agar. It is a gelatinous polysaccharide extracted from red algae, which is a promising biopolymer for the development of biodegradable packaging [7]. Its main chain consists of 1,3-linked D-galactose and 1,4-linked 3,6-anhydro-L-galactose units [8].
Due to their biodegradability and gelling properties, flaxseed gum and agar are biopolymers of great interest for the development of bioplastic films. Despite the large amount of biopolymers already existing, the development of new blends deserves to be explored. Blends are alternative biopolymer modifications that could result in improved properties.
Compatibility is used to describe polymer blends which result in a (preferably commercially) desirable set of properties [9]. Garrido and collaborators studied soy agar/protein-based blends that showed good compatibility and decreased solubility with agar incorporation [10]. In another work, Hussain studied rice starch blends with flaxseed gum. The properties for the gels, in the presence of gum, showed greater hardness and adhesiveness values, in addition to an increase in the system viscosity [11]. To the best of our knowledge, there is no study in the literature about blends and nanocomposites of flaxseed gum and agar films reinforced with cellulose nanocrystals (CNC).
Flaxseed and agar films are fragile and brittle, therefore, they are difficult to process, which makes their application as unviable bioplastics. Furthermore, it becomes necessary to add plasticizers and crosslinking agents. Plasticizers act to reduce intermolecular interactions, increasing the distances between the polymeric chains and, consequently, increasing the flexibility [12]. Glycerol is one of the plasticizers mostly used because it has good compatibility with hydrophilic biomacromolecules [13]. Glutaraldehyde is used as a crosslinking agent to produce better mechanical strength [14], in addition to improving the resistance of biopolymers in water [15]. Glutaraldehyde is a low-cost crosslinking agent with high reactivity [16], reported in the development of films and blends of biopolymers [14, 16, 17].
Cellulose nanocrystals (CNC) are also within the scope of biopolymers, and are used as reinforcing agents in particular due to their high aspect ratio, biocompatibility, biodegradability and high elastic modulus. All these characteristics contribute to improve performance with respect to mechanical properties. In addition, the large amount of hydroxyl groups (–OH) on their surface makes them compatible with hydrophilic polymers [18].
The objective of this work was to investigate the addition of agar to flaxseed gum blends in the presence of glutaraldehyde (crosslinking agent) and glycerol (plasticizer). The incorporation of different contents (2, 4 and 8% by wt) of CNCs in the polymeric blends was also evaluated. Films, blends and nanocomposites were produced by casting, and then were characterized by thermal analysis (thermal gravimetric analysis and differential scanning calorimetry) and spectroscopy. The interaction with water (vapor permeability and solubility), scanning electron microscopy and tensile tests were also performed. To the best of our knowledge, there is no record in the literature about studies of FG/AG blends reinforced with CNC.
Experimental
Materials
Flaxseed golden grains were purchased from a local supermarket. The other materials were agar (Isofar), phosphorus pentoxide (P2O5, 98.5%; Sigma Aldrich), glycerol (Isofar), glutaraldehyde solution (50% by wt in H2O; Sigma Aldrich), hydrochloric acid (Vetec), chloride potassium (99–100%; Vetec), Kraft pulp (Conpacel Company) and sulfuric acid (95–98%; Vetec).
Preparation of cellulose nanocrystals
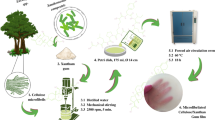
CNCs were isolated from Kraft pulp of Eucalyptus urograndis (a hybrid of Eucalyptus urophila and Eucalyptus grandis). The experimental conditions for obtaining CNCswere conducted in accordance with da Silva and collaborators [19]. The CNCs were prepared by hydrolysis at 45 °C for 50 min using 20 mL of H2SO4 solution with 60% by wt per gram of fiber.
Extraction of flaxseed gum
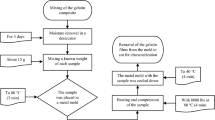
For extraction of the flaxseed gum, 240 g of seeds was soaked in distilled water (960 mL) at room temperature (25 ± 1 °C) for around 18 h. After this time, additional distilled water (800 mL) was added, and the mixture was vigorously stirred for 15 min [20]. Finally, two filtrations using cloth filter were carried out to separate the gum from the seeds. The soluble fraction was collected and used without purification (in natura form) to prepare the blends and nanocomposite films.
Preparation of films and blends
The neat flaxseed gum (FG) films were made using only the gum suspension (FGsuspension) calculated assuming 2.0 g of total dry mass in the formulation (FGdry). Film control was produced using the formulation reportedin our previous work [20]. For the control film and blends, the pH was adjusted to 3.5 by adding HCl solution (1.0 mol/L) to the polymer suspensions. The amounts of glycerol (GLY) and glutaraldehyde (GLA) were fixed at 30% w/w and 15% w/w.
For the blends preparation, agar (AG) was dissolved in distilled water in the quantities and proportions described in Table 1. This mixture was heated to 95 °C. The solution was kept at that temperature under constant stirring for 2 min and then immediately mixed with the suspension of gum and additives, and premixed with stirring for 10 min. The mixture was stirred without heating for a further 5 min. The different formulations were named 75/25, 50/50 and 25/75, according to the percentage of FG/AG in the formulation (Table 1).
All films were prepared using a casting method in Petri dishes with dimensions of 150 mm × 15 mm at 35 °C for 48 h in an air circulating oven (Jeio Tech model OF-02).
Preparation of nanocomposites
Nanocomposites were obtained from the blended formulations by the addition of 2, 4 and 8% w/w CNCs, which were added together with the gum suspension and additives. Paralikar and collaborators showed that when 10% w/w of CNCs is incorporated in hydrophilic matrices, agglomeration can occur [21]. Thus, in this work, the 8% w/w value was the higher content of CNC employed. The other percentages were reduced proportionally, with the aim of evaluating its effect in the samples obtained. The nanocomposite samples were labeled FG/AG-2% CNC, FG/AG-4% CNC and FG/AG-8% CNC, according to the amount of CNCs added to each blend formulation (FG/AG = 75/25, 50/50 and 25/75).
Attenuated total reflectance with Fourier transform infrared spectroscopy (ATR-FTIR)
ATR-FTIR experiments were performed on a Shimadzu IRPrestige-21 equipment and the transmittance spectra of the films and blends were obtained. The experiments were carried out in the range of 650–4000 cm−1, with a resolution of 4 cm−1 and a total of 32 scans for each sample.
Thermal gravimetric analysis (TGA)
The thermal stability of the films and blends was evaluated using a Shimadzu DTG-60H equipment. The analysis conditions were: a nitrogen atmosphere with a flow rate of 50 mL/min, a heating rate of 10 °C/min, a temperature range of 25 to 600 °C and sample mass of 6 ± 0.001 mg.
Differential scanning calorimetry (DSC)
The thermal analyses for all films and blends were performed using a DSC Q20 differential scanning calorimeter (TA Instruments). Around 5 mg of each sample was taken into an aluminum pan and heated from 25 to 230 °C at a heating rate of 10 °C/min with a nitrogen flow of 50 mL/min.
Atomic force microscopy (AFM)
AFM was analyzed using a Shimadzu SPM-9600. A droplet of the aqueous CNC suspension (1 × 10–4 g/mL) was deposited onto a freshly cleaved mica surface and air-dried. AFM images were captured with a scan rate of 1 Hz using Si tips with a curvature radius of less than 10 nm and a spring constant of 42 nm−1. The dimensions of the nanocrystals were determined usingVectorScan software.
Solubility
The solubity of each sample was measured according to ASTM D 870-2. Three aliquots of each film formulation, blend and nanocomposite (diameter of 2 cm) were dried in an oven at 105 °C for 2 h. After that, they were weighed to determine the initial dry matter of each film (\({w}_{i}\)), and then immersed into 30 mL of distilled water in a beaker. A volume of 30 mL was sufficient for total immersion of the sample into solvent. The beaker was sealed and periodically stirred up to 24 h at 23 ± 2 °C. Finally, the insoluble portion of the sample was removed, oven dried at 105 °C for 24 h and weighed (\({w}_{f}\)) to determine the weight of the solubilized dry matter. The water solubility (S) of each film was determined by Eq. 1:
Water vapor permeability
Permeation to water vapor was performed according to ASTM E96–E95, using Payne’s cup technique with P2O5 as a drying agent. This analysis was done considering the mass loss of the permeant (water), which can pass through the film as a function of time and the permeation area (water vapor permeance). Thus, the permeability was obtained from the water vapor permeance of the unit thickness induced by the unit vapor pressure difference between two specific surfaces. Two replicates of each sample were placed in a controlled environmental chamber with a constant temperature of 20 ± 1.0 °C and 0% relative humidity.
Scanning electron microscopy (SEM)
The surface and fracturing of samples were analyzed with a scanning electron microscope, TECAN model Vega 3. The samples were subjected to fracture after immersion in liquid nitrogen and subsequently fixed on aluminum supports with carbon tape and metallized with gold. SEM images were obtained with a 3.5 kX magnification and an acceleration voltage of 5.0 kV.
Tensile tests
The tensile behavior of the samples was analyzed using an Instron 5982 universal testing machine with a 5 kN load cell according to ASTM D 882-02. The experiments were performed at room temperature with a crosshead speed of 25 mm/min upon rectangular strips (1 × 9 cm2). The distance between the claws was 5 cm, and the thickness was measured before each measurement. Before the analysis, the samples were conditioned at 25 ± 2 °C for 3 days in controlled relative humidity (59% RH—sodium bromide solution). The tensile strength-at-break (σ), elongation-at-break (ε), Young’s modulus (E) and tenacity (T) were determined from these samples. Ten samples were assayed for each formulation. The thickness of the samples was measured using a micrometer (Zaas precision) and the values are reported as the average measurments from five aleatory points into selected films area.
Statistical analysis
The data were submitted to statistical analysis by Analysis of Variance (ANOVA), using the Statistica®software, version 8.0 (StatSoft, South America). Duncan’s test was used to determine differences at a level of significance of 5% (p ≤ 0.05).
Results and discussion
ATR-FTIR analysis
The FTIR spectra of the films and blends are shown in Fig. 1. The major peak at ~ 3357 cm−1 was due to the –OH stretching vibration. The peak at 1620 cm−1 in the FG is ascribed to the C=O stretching vibrations of galacturonic acid and the presence of water, whereas –CH2 scissoring and –OH bending vibrations provided absorbance lines of 1414 cm−1 [22]. The peaks gradually weakened with the decrease of FG in the films and blends. The absorption band at 1149 cm−1 corresponding to the glycoside bond C–O–C [23], presented for the FGcontrol suggests increased C–O–C bonds between polysaccharide chains due to the addition of the crosslinking agent. The peak at 1070 and 930 cm−1 associated with the 3,6-anhydro-galactose bridges [24], was gradually intensified with the increase in AG.
Thermal study
Knowledge of the thermal properties of biopolymers is fundamental for the definition of processing conditions and applications, and is also necessary to evaluate the interactions between the matrix and additives.
The TGA graphs are shown in Fig. 2. The initial weight loss for all films was observed at ~ 90 °C, which is justified by the removal of adsorbed water in the films. Subsequent degradation steps varied depending on the formulation, which was attributed to the complex degradation processes of the macromolecular chains of AG and FG, as well as the additives degradation, which induced a decrease in thermal stability over the pure FG film, as verified in Table 2.
The high amount of these additives (glycerol and glutaraldehyde) yielded a reduction in the thermal stability. The agar addition did not influence the thermal stability relative to FGcontrol, since the initial temperatures of degradation were not significantly modified with the agar incorporation, as can be seen in Fig. 2.
The DSC graphs are shown in Fig. 3. The enthalpy values and maximum temperature estimation associated to the event and endothermic peak profile were calculated using the TA Analysis software. The films and blends exhibited an endothermic peak with a maximum temperature upward of 100 °C. The presence of this phenomenon could be attributed to a loss of water and is associated with the macromolecular organization due to solvent evaporation during the film preparation process. Water plays an important role in the hydrophilic polymeric films and can change their thermal properties.
The high additive incorporation in the FG films resulted in a decrease in enthalpy values (Table 2). This reveals that films vary in their water-holding capacity. Therefore, the incorporation of glutaraldehyde resulted in a film with less water content due to the crosslinking effect.
As can also be observed when different amounts of agar (25, 50 and 75% w/w) were added to the blend formulations, the enthalpy values increased by 23.3, 23.7 and 34.5% compared to the FGcontrol sample, respectively. Therefore, the addition of agar results in a higher water content compared to the FGcontrol.
AFM analysis
The AFM images in Fig. 4 show the presence of isolated CNCs in the nanometer scale. The CNCs display a rod-like shape with an average length of 155.37 ± 26.97 nm and an average diameter of 4.29 ± 1.03 nm. The nanocrystal dimension agrees with earlier reports of CNC prepared with the same isolation process by da Silva and collaborators [19] who reported an average length of 175.7 ± 63.4 nm and an average diameter of 4.63 ± 1.36 nm.
Interaction of films and nanocomposites with water
The hydrophilicity of biopolymers is a relevant parameter for bioplastic applications. Some applications may require low hydrophilicity to maintain product integrity [25], but other applications may require prior hydration, like seed coatings for the controlled application of fertilizers.
Water solubility
The water solubility of the nanocomposite films is shown in Table 3. The FG films were completely soluble in water, however, for the FGcontrol sample, the film showed a significant reduction from 100 to 52.6%, which may be related to the crosslinking process due to the addition of a crosslinking agent [20].
For the blends, a small but a significant (p < 0.05) reduction in solubility was observed with the incorporation of agar. Garrido and collaborators [10] also reported a reduction in solubility with agar incorporation in soy protein films.
All blends showed a significant increase (p < 0.05) in solubility with the addition of CNCs. However, when a higher amount of CNCs was added, there was a decrease in solubility. The reduction in solubility is indicative of strong interactions established in formulations with higher CNC contents in the matrix [26], suggesting the formation of a three-dimensional network formed by hydrogen bonds between CNCs [27].
Water vapour permeability
The barrier properties of the films against water vapor are shown in Table 3. Only the FG sample was too fragile to complete the test. The incorporation of 25% agar (75/25) induced a significant drop (p < 0.05) in permeability. In contrast, high proportions of agar generated an increase in the permeant passage through the matrix.
This behavior can be explained because the agar presents high moisture sensitivity [28], despite showing moderate water resistance [29]. High moisture sensitivity contributes to greater water uptake and a consequent increase in Pw, as evidenced by samples containing a higher proportion of agar.
The incorporation of 4% and 8% CNCs has no significant (p > 0.05) effect on the Pw of the 50/50 blend. However, the addition of CNCs provided an increase in Pw for the 75/25 nanocomposite formulations. Atef and collaborators [30] also reported an increase in Pw values in the agar film with the addition of 2.5 and 5% CNCs, but a decrease of Pw with the incorporation of 10% of CNCs. In this work, for the nanocomposites with the highest agar percentage (25/75), there was a reduction in Pw with the incorporation of 2% and 4% CNCs and an increase with the addition of 8% CNCs. No relationship was observed between solubility and Pw. However, three mechanisms involved in Pw must be considered: water vapour absorption at the film surface, diffusion of water vapor through the film and water vapor desorption from the surface of the film [10].
SEM analysis of morphology
SEM analysis of the FG/AG films was performed to observe agar dispersion in the flaxseed gum matrix (Fig. 5). Previously, Phan and collaborators [8] presented micrographs of agar films and confirmed dense and homogeneous structures. As can be seen here, the film FGcontrol presented an uneven surface that may have been due to the glycerol exudation on the surface of the film, as discussed in our previous work [20].
This exudation was macroscopically evidenced by the sticky aspect of the film. It is possible to verify that the incorporation of agar to the FGcontrol films caused a minimization in the glycerol exudation effect, as well as a more compact structure. These observations again confirm the homogeneity of the FG, AG and additives.
The blends exhibited a rougher appearance as the amount of agar in the formulation increased. The dense and uniform structure of the fracture region (Fig. 6) confirmed good aggregation of the molecules during the solvent evaporation, even with higher CNC contents, showing a dense structure and high homogeneity.
Tensile test
Table 4 shows the results obtained for the maximum stress at rupture, elongation-at-upture, Young’s modulus and toughness for the FGcontrol, blends and nanocomposites.
The maximum stress at rupture (σ) is the resistance offered by the material at the point of rupture. The elongation-at-rupture (ε) gives information on how the material can deform before rupture. The modulus of elasticity, also known as Young’s modulus (E), is an indicator of film stiffness. The larger the modulus, the smaller the elastic deformation resulting from the application of tension.
These measurements were not performed for FG because of its fragility. The control film of flaxseed gum presented a reduced σ but a high value of ε. Tee and collaborators [31] showed that with increasing glycerol concentration the ε of the films significantly increased, whereas the E and σ values decreased. Seeds gum-based films with desired mechanicalcharacteristics can be obtained by adjusting glycerol content [32]. Tee and collaborators [31] studied the mechanical properties of flaxseed gum with glycerol in basic pH (≅ 9.0), but without glutaraldehyde addition. They found lower values of σ compared to those obtained in this work (film control, pH ≅ 3.5).
The mechanical test results for the blends presented in Table 4 show that the incorporation of agar increased the tensile strength of the flaxseed gum films, resulting in more resistant films. Jumaidin and collaborators [33] reported an increase in the thermoplastic starch tensile properties with the incorporation of agar.
In view of the results obtained (Table 4), it is desirable that the incorporation of agar into the polymeric blends formulations contributes to an improvement in the mechanical properties (evidenced in terms ofthe E values). However, improvement in terms of T is also desirable, since handling films with high rigidity is difficult. A study was conducted to select the better content of agar that could be incorporated to provide these properties. The formulation 25/75 was the one that stood out the most between the blends, because it was observed the percentage increased by 3272% and 811% in the E and T values, respectively. Similar results were obtained by Fekete and collaborators when the agar considerably increased the stiffness and strength of thermoplastic starch [34].
The CNC incorporation was conducted with the aim of improving the mechanical properties, due to the characteristics of high reinforcement capacity at low load levels [35]. When CNCs were incorporated into 75/25 formulation, there was no significant improvement (p > 0.05) in mechanical properties, as evidenced by E values. The nanocomposites of 25/75 showed a significant improvement (p > 0.05) in the mechanical properties, as evidenced by E values, only with 4% and 8% w/w CNCs.
The highest values of E among all formulations were obtained for the nanocomposites 50/50–2% CNC and 50/50–4% CNC. The increase in E values of the CNC-reinforced blends can be attributed to the increase in stiffness by the addition of CNCs [36].
Tenacity measures the amount of energy a material that can absorb before fracture [37]. It can be seen that the control film of flaxseed gum had a smaller T. The 75/25, 50/50 and 25/75 blends presented higher values of T of 585%, 636% and 811%, respectively, relative to FGcontrol.
The samples that presented higher E and high tenacity were the formulations 50/50–2% CNC and 50/50–4% CNC, which showed a significant increase (p > 0.05) in T of 130% compared to the 50/50 blend. The best formulation of nanocomposites was 50/50–4% CNC, that presented higher E and increase in tenacity of 1588% relative to the control film. Therefore, improvements in mechanical properties could be seen both in the blends and the nanocomposites.
Conclusion
Nanocomposites obtained from FG/AG blends are possible alternatives that can improve the properties of FG. The different formulations of blends and nanocomposites presented improvements in the mechanical properties and solubility, showing satisfactory results for the blends and nanocomposite formulations for application as bioplastics. All the formulations of blends presented increases of tenacity between 585 and 811% and lower values of solubility relative to the control film (FGcontrol). The best formulation of nanocomposites was 50/50–4% CNC, that presented higher modulus of elasticity and increase in tenacity of 130% relative to the 50/50 blend and 1588% relative to the control film. These samples are candidates for applications like packaging, which requires a greater amount of energy before breaking and higher stiffness.
References
Verbeek CJR, Bier JM (2011) Synthesis and characterization of thermoplastic agro-polymers. RSC publishing, New Zealand
Beikzadeh S, Khezerlou A, Jafari SM, Pilevar Z, Mortazavian AM (2020) Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv Colloid Interface Sci 280:102164
Mostafavi FS, Zaeim D (2020) Agar-based edible films for food packaging applications—a review. Int J Biol Macromol 159:1165–1176
Khalloufi S, Corredig M, Goff HD, Alexander M (2009) Flaxseed gums and their adsorption on whey protein-stabilized oil-in-water emulsions. Food Hydrocoll 23:611–618
Warrand J, Michaud P, Picton L, Muller G, Courtois B, Ralainirina R, Courtois J (2005) Structural investigations of the neutral polysaccharide of Linum usitatissimum L. seeds mucilage. Int J Biol Macromol 35:121–125
Muralikrishna G, Salimath PV, Tharanathan RN (1987) Structural features of an arabinoxylan and a rhamno-galacturonan derived from linseed mucilage. Carbohydr Res 161:265–271
Kanmani P, Rhim JW (2014) Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr Polym 102:708–716
Phan TD, Debeaufort F, Luu D, Voilley A (2005) Functional properties of edible agar-based and starch-based films for food quality preservation. J Agric Food Chem 53:973–981
Folkes MJ, Hope PS (1993) Polymer blends and alloys. Springer, Dordrecht
Garrido T, Etxabide A, Guerrero P, Caba K (2016) Characterization of agar/soy protein biocomposite films: effect of agar on the extruded pellets and compression moulded films. Carbohydr Polym 151:408–416
Hussain S (2016) Native rice starch and linseed gum blends: effect on the pasting, thermal and rheological properties. Czech J Food Sci 33:556–563
McHugh TH, Krochta JM (1994) Sorbitol- vs glycerol-plasticized whey protein edible films: integrated oxygen permeability and tensile property evaluation. J Agric Food Chem 42:841–845
Cervera MF, Karjalainen M, Airaksinen S, Rantanen J, Krogars K, Heinämäki J, Colarte AI, Yliruusi J (2004) Physical stability and moisture sorption of aqueous chitosan-amylose starch films plasticized with polyols. Eur J Pharm Biopharm 58:69–76
Won JS, Lee TS, Kim HS, Son HG, Hong TM, Lee HW, Lee SG (2014) Preparation and characterization of kenaf/soy protein biocomposites. J Biobased Mater Bioenergy 8:221–229
Liu L, Liu CK, Fishman ML, Hicks KB (2007) Composite films from pectin and fish skin gelatin or soybean flour protein. J Agric Food Chem 55:2349–2355
Sen C, Das M (2017) Self-supporting-film from starch, poly(vinyl alcohol), and glutaraldehyde: optimization of composition using response surface methodology. J Appl Polym Sci 134:44436
Prakash N, Arungalai VS (2016) Biodegradable polymer based ternary blends for removal of trace metals from simulated industrial wastewater. Int J Biol Macromol 83:198–208
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J, Osterberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindstrom T, Hadjichristidis N, Faul CFJ, Wild PM, Abe K, Nogi M, Nakagaito AN, Mangalam A, Simonsen J, Benight AS, Bismarck A, Berglund LA, Peijs T (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941
Silva ISV, Neto WPF, Silvério HA, Pasquini D, Andrade MZ, Otaguro H (2015) Mechanical, thermal and barrier properties of pectin/cellulose nanocrystal nanocomposite films and their effect on the storability of strawberries (Fragaria ananassa). Polym Adv Technol 28:1005–1012
Prado NS, Silva ISV, Silva TAL, Oliveira WJ, Motta LAC, Pasquini D, Otaguro H (2018) Nanocomposite films based on flaxseed gum and cellulose nanocrystals. Mater Res 21:20180134
Paralikar SA, Simonsen J, Lombardi J (2008) Poly(vinyl alcohol)/cellulose nanocrystal barrier membranes. J Memb Sci 320:248–258
Liu J, Shen J, Shim YY, Reaney MJT (2016) Carboxymethyl derivatives of flaxseed (Linum usitatissimum L.) gum: characterisation and solution rheology. Int J Food Sci Technol 51:530–541
Pawlak A, Mucha M (2003) Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta 396:153–166
El-hefian EA, Nasef MM, Yahaya AH (2012) Preparation and characterization of chitosan/agar blended films: part 1. chemical structure and morphology. E-J Chem 9:1431–1439
Mukurumbira AR, Mellem JJ, Amonsou EO (2017) Effects of amadumbe starch nanocrystals on the physicochemical properties of starch biocomposite films. Carbohydr Polym 165:142–148
Slavutsky AM, Bertuzzi MA (2014) Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr Polym 110:53–61
Rodriguez GNL, Thielemans W, Dufresne A (2006) Sisal cellulose whiskers reinforced polyvinyl acetate nanocomposites. Cellulose 13:261–270
Sousa AMM, Souza HKS, Latona N, Liu CK, Gonçalves MP, Liu L (2014) Choline chloride based ionic liquid analogues as tool for the fabrication of agar films with improved mechanical properties. Carbohydr Polym 111:206–214
Giménez B, Lacey AL, Pérez-Santín E, López-Caballero ME, Montero P (2013) Release of active compounds from agar and agar–gelatin films with green tea extract. Food Hydrocoll 30:264–271
Atef M, Rezaei M, Behrooz R (2014) Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. Int J Biol Macromol 70:537–544
Tee YB, Wong J, Tan MC, Talib RA (2016) Development of edible film from flaxseed mucilage. BioResources 11:10286–10295
Salehi F (2019) Characterization of new biodegradable edible films and coatings based on seeds gum: a review. J Packag Technol Res 3:193–201
Jumaidin R, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) Characteristics of thermoplastic sugar palm starch/agar blend: thermal, tensile, and physical properties. Int J Biol Macromol 89:575–581
Fekete E, Bella É, Csiszár E, Móczó J (2019) Improving physical properties and retrogradation of thermoplastic starch by incorporating agar. Int J Biol Macromol 136:1026–1033
Siqueira G, Dufresne BJA (2010) Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers 2:728–765
Khan A, Khan RA, Salmieri S, Tien CL, Riedl B, Bouchard J, Chauve G, Tan V, Kamal MR, Lacroix M (2012) Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr Polym 90:1601–1608
Lorevice MV, de Moura MR, Aouada FA, Mattoso LHC (2012) Development of novel guava puree films containing chitosan nanoparticles. J Nanosci Nanotechnol 12:2711–2717
Acknowledgements
The authors thank Coordination for the Improvement of Higher Education Personnel (CAPES), the National Counsel of Technological and Scientific Development (CNPq) and Federal University of Uberlândia for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prado, N.S., da Silva, I.S.V., de Almeida Nascimento, J.A. et al. Flaxseed gum/agar blends and nanocomposites: preparation and physical properties. Iran Polym J 30, 821–830 (2021). https://doi.org/10.1007/s13726-021-00933-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-021-00933-w