Abstract
Purpose of Review
Actinic keratoses (AKs) are foci of dysplastic keratinocytes in the epidermis. They are markers of both photodamage and risk of keratinocyte carcinoma (KC) formation. Individual AKs are thought to progress to KC uncommonly, although they arise from the same UV-induced mutations which give rise to KC. Thus, the rationale for treatment of AKs is to reduce the risk of KC formation since AKs are not dangerous in themselves. An estimated $1.68 billion is spent on treatment of AKs in the United States annually. Treatment of a region of skin (e.g., face, scalp, or forearms) to clear AKs is termed field treatment. Though field treatment of AKs and actinic damage has been associated with a reduction in cutaneous squamous cell carcinoma (CSCC), it is unclear to what degree treatment of individual lesions positively impacts health.
Recent Findings
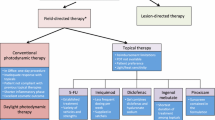
The presence of AKs has been correlated with formation of KC, particularly CSCC. Field treatment can be accomplished by topical therapy [photodynamic therapy (PDT), topical 5-fluorouracil, ingenol mebutate, imiquimod, and/or sunscreen] or oral systemic therapy including nicotinamide or retinoids (i.e., acitretin, isotretinoin). Field treatments have been associated with a reduction in KC and therefore are likely cost-effective. However, there is a lack of data showing that treatment of individual AK lesions (usually accomplished via cryotherapy) reduces KC. Although cryotherapy is only associated with a 4% sustained clearance rate at 1 year, more than three times the amount of money is spent on cryotherapy as compared to topical therapy in Medicare patients.
Summary
Field treatment is superior to cryotherapy for reduction of KC. However, it requires high patient compliance and increasingly entails high out-of-pocket expenses for patients. Since field-directed and systemic therapies of AKs reduce KC formation, allocation of resources should be directed toward these modalities. Efforts should be made to improve patient access to field treatments by increased reimbursement for photodynamic therapy (both standard and daylight), reduction of out-of-pocket expenses for topical treatments and retinoids, and increased use of nicotinamide in patients with field actinic damage.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Weinstock MA, Bingham SF, Cole GW, Eilers D, Naylor MF, Kalivas J, et al. Reliability of counting actinic keratoses before and after brief consensus discussion: the VA Topical Tretinoin Chemoprevention (VATTC) trial. Arch Dermatol. 2001;137:1055–8.
Callen JP, Bickers DR, Moy RL. Actinic keratoses. J Am Acad Dermatol. 1997;36:650–3.
Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1:795–7.
Quaedvlieg PJ, Tirsi E, Thissen MR, Krekels GA. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335–9.
Criscione VD, Weinstock MA, Naylor MF, Luque C, Eide MJ, Bingham SF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523–30.
Werner RN, Sammain A, Erdmann R, Hartmann V, Stockfleth E, Nast A. The natural history of actinic keratosis: a systematic review. Br J Dermatol. 2013;169:502–18.
Berman B, Cockerell CJ. Pathobiology of actinic keratosis: ultraviolet-dependent keratinocyte proliferation. J Am Acad Dermatol. 2013;68:S10–9.
South AP, Purdie KJ, Watt SA, Haldenby S, den Breems NY, Dimon M, et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol. 2014;134:2630–8.
• Lim HW, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958–972 e952. Prevanlence, cost, and mortality analysis of 24 skin disease categories in the US population using private and government claims data.
Lim HW, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151–1160 e1121.
Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42:1041–7.
Brin L, Zubair AS, Brewer JD. Optimal management of skin cancer in immunosuppressed patients. Am J Clin Dermatol. 2014;15:339–56.
Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–9.
• Levine DE, Karia PS, Schmults CD. Outcomes of patients with multiple cutaneous squamous cell carcinomas: a 10-year single-institution cohort study. JAMA Dermatol. 2015;151:1220–5. A retrospective cohort study of primary CSCC found that the 10-year cumulative incidence of local recurrence and nodal metastasis is higher in patients with 2 to 9 CSCCs and markedly higher in those with 10 or more tumors compared with patients with a history of 1 CSCC.
Toro JR, Blake PW, Bjorkholm M, Kristinsson SY, Wang Z, Landgren O. Prior history of non-melanoma skin cancer is associated with increased mortality in patients with chronic lymphocytic leukemia. Haematologica. 2009;94:1460–4.
Xiong MY, Rizzo AE, Cohen TSD, Dyer RK, Korgavkar K, Bingham SF, et al. Predictors of squamous cell carcinoma in high-risk patients in the VATTC trial. J Invest Dermatol. 2013;133:1521–32.
Ianhez M, Miot HA, Bagatin E. Liquid nitrogen for the treatment of actinic keratosis: a longitudinal assessment. Cryobiology. 2014;69:140–3.
Krawtchenko N, Roewert-Huber J, Ulrich M, Mann I, Sterry W, Stockfleth E. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(Suppl 2):34–40.
Werner RN, Jacobs A, Rosumeck S, Erdmann R, Sporbeck B, Nast A. Methods and Results Report—Evidence and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum. J Eur Acad Dermatol Venereol. 2015;29:e1–66.
Drew BA, Karia PS, Mora AN, Liang CA, Schmults CD. Treatment patterns, outcomes, and patient satisfaction of primary epidermally limited nonmelanoma skin cancer. Dermatol Surg. 2017;43:1423–30.
Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M, et al. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or fluorouracil for treatment of squamous cell carcinoma in situ: results of a multicenter randomized trial. Arch Dermatol. 2006;142:729–35.
Overmark M, Koskenmies S, Pitkanen S. A retrospective study of treatment of squamous cell carcinoma in situ. Acta Derm Venereol. 2016;96:64–7.
Cozzi SJ, Ogbourne SM, James C, Rebel HG, de Gruijl FR, Ferguson B, et al. Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol. 2012;132:1263–71.
Dragieva G, Prinz BM, Hafner J, Dummer R, Burg G, Binswanger U, et al. A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratoses in transplant recipients. Br J Dermatol. 2004;151:196–200.
• Pomerantz H, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952–60. A double-blind randomized placebo-controlled trial of 5% fluorouracil cream twice a day for 4 weeks compared to vehicle showed that the fluorouracil group had significantly fewer AKs at 6 months and 2.6 years.
Swanson N, Smith CC, Kaur M, Goldenberg G. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3 multicenter, randomized, double-blind, placebo-controlled studies. J Drugs Dermatol. 2013;12:1278–82.
Stockfleth E, Gupta G, Peris K, Aractingi S, Dakovic R, Alomar A. Reduction in lesions from Lmax: a new concept for assessing efficacy of field-directed therapy for actinic keratosis. Results with imiquimod 3.75. Eur J Dermatol. 2014;24:23–7.
• Weinstock MA TS, Siegel JA, Marcolivio K, Means AD, Leader NF, Shaw FM, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018; https://doi.org/10.1001/jamadermatol.2017.3631. A double-blind randomized placebo-controlled trial of 5% fluorouracil cream twice a day for 2 or 4 weeks compared to vehicle found a 75% risk reduction in CSCC formation in the fluoruracil group.
Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg. 2010;36:652–8.
• Ulrich C, et al. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case-control study. Br J Dermatol. 2009;161(Suppl 3):78–84. A matched prospective cohort study of regular sunscreen use in organ transplant recipients found reduction in AKs and CSCC in the sunscreen group.
Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–702.
Kadakia KC, Barton DL, Loprinzi CL, Sloan JA, Otley CC, Diekmann BB, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128–37.
de Sevaux RG, Smit JV, de Jong EM, van de Kerkhof PC, Hoitsma AJ. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol. 2003;49:407–12.
Bavinck JN, Tieben LM, van der Woude FJ, Tegzess AM, Hermans J, ter Schegget J, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933–8.
George R, Weightman W, Russ GR, Bannister KM, Mathew TH. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269–73.
Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618–26.
Chen AC, Martin AJ, Dalziell RA, McKenzie CA, Lowe PM, Eris JM, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016;175:1073–5.
Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421–6.
Tennvall GR, Norlin JM, Malmberg I, Erlendsson AM, Haedersdal M. Health related quality of life in patients with actinic keratosis—an observational study of patients treated in dermatology specialist care in Denmark. Health Qual Life Outcomes. 2015;13:111.
Weinstock MA, Lee KC, Chren MM, Marcolivio K, V. T. Group. Quality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) Trial. J Am Acad Dermatol. 2009;61:207–15.
Lee K, Weinstock M. Prospective quality of life impact of actinic keratoses: observations from the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Acta Derm Venereol. 2011;91:101–2.
Jubert-Esteve E, del Pozo-Hernando L, Izquierdo-Herce N, Bauzá-Alonso A, Martín-Santiago A, Jones-Caballero M. Quality of life and side effects in patients with actinic keratosis treated with ingenol mebutate: a pilot study. Actas Dermosifiliogr. 2015;106:644–50.
Esmann S, Vinding GR, Christensen KB, Jemec GB. Assessing the influence of actinic keratosis on patients’ quality of life: the AKQoL questionnaire. Br J Dermatol. 2013;168:277–83.
Song H, Adamson AS, Mostaghimi A. Trends in Medicare spending on topical immunomodulators and chemotherapies. J Am Acad Dermatol. 2017. https://doi.org/10.1016/j.jaad.2017.07.056.
Adamson AS, Dusetzina SB. Characteristics of Medicare payments to dermatologists in 2013. JAMA Dermatol. 2017;153:95–7.
Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32:327–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Ruiz, E.S., Schmults, C.D. Risk Stratification: Should All Actinic Keratoses in All Patients Be Treated?. Curr Derm Rep 7, 99–104 (2018). https://doi.org/10.1007/s13671-018-0217-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-018-0217-x