Abstract
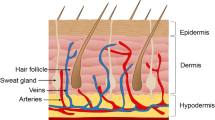
Wounds created by skin cancer surgery often heal by secondary intention. Many novel options to assist in wound healing exist. This article reviews the biology of wound healing, indications for healing by secondary intention, types of dressings, categories, and brand names of tissue-engineered skin substitutes, as well as other wound healing modalities. The cost-benefit analysis must be considered given the expense of these newer technologies.
Similar content being viewed by others
References
Papers of particular interest, published recently have been highlighted as: • Of importance •• Of major importance
Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18.
Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46.
Stojadinovic A, Carlson JW, Schultz GS, Davis TA, Elster EA. Topical advances in wound care. Gynecol Oncol. 2008;111(2 Suppl):S70–80.
Lawrence W, Diegelmann R. Growth factors in wound healing. Clin Dermatol. 1994;12:157–69.
Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care. 2014;3(8):511–29. Review article summarizing available data for wound dressings.
Zitelli JA. Wound healing by secondary intention: a cosmetic appraisal. J Am Acad Dermatol. 1983;9(3):407–15.
Donaldson MR, Coldiron BM. Scars after second intention healing. Facial Plast Surg. 2012;28(05):497–503.
Ovington LG. Hanging wet to dry dressings out to dry. Home Healthc Nurse. 2001;19:477.
Ubbink DT, Vermeulen H, van Hattem J. Comparison of homecare costs of local wound care in surgical patients randomized between occlusive and gauze dressings. J Clin Nurs. 2008;17(5):593–601.
Rubio PA. Use of semiocclusive, transparent film dressings for surgical wound protection: experience in 3637 cases. Int Surg. 1991;76(4):253–4.
Hien NT, Prawer SE, Katz HI. Facilitated wound healing using transparent film dressing following Mohs micrographic surgery. Arch Dermatol. 1988;124(6):903–6.
Takeuchi A, Tsuchiya H, Shirai T, Hayashi K, Nishida H, Tomita K. Occlusive dressing for large soft tissue defects following soft tissue tumor excision. J Orthop Sci. 2009;14(4):385–90.
Eisenbud D, Hunter H, Kessler L, Zulkowski K. Hydrogel wound dressings: where do we stand in 2003? Ostomy Wound Manage. 2003;49:52–7.
Galli MM, Protzman NM, Brigido SA. Utilization of silver hydrogel sheet dressing on postsurgical incisions. Foot Ankle Spec. 2013;6(6):422–33.
Agren MS, Mertz PM, Franzén L. A comparative study of three occlusive dressings in the treatment of full-thickness wounds in pigs. J Am Acad Dermatol. 1997;36(1):53–8.
Nguyen CV, Washington CV, Soon SL. Hydrocolloid dressings promote granulation tissue on exposed bone. Dermatol Surg. 2013;39(1):123–5.
von Lindern JJ, Niderbagen B, Appel T, Stefaan B. Treatment of soft tissue defects with exposed bone in the head and face region with alginates and hydrocolloid dressings. J Oral Maxillofac Surg. 2002;60(10):1126–30.
Daiser D, Hafner J, Mayer D, French LE, Lauchli S. Alginate dressing and polyurethane film versus paraffin gauze in the treatment of split-thickness skin graft donor sites: a randomized controlled pilot study. Adv Skin Wound Care. 2013;26(2):67–73.
Barnea Y, Amir A, Leshem D, Zaretski A, Weiss J, Shafir R, et al. Clinical comparative study of aquacel and paraffin gauze dressing for split-skin donor site treatment. Ann Plast Surg. 2004;53(2):132–6.
Perkins K, Davey RB, Wallis KA. Silicone gel: a new treatment for burn scars and contractures. Burns. 1983;9:201.
de Oliveira GV, Nunes TA, Magna LA, Cintra ML, Kitten GT, Zarpellon S, et al. Silicone versus nonsilicone gel dressings, a controlled trial. Dermatol Surg. 2001;27(8):721–6.
Li-Tsang CW, Lau JC, Choi J, Chan CC, Jianan L. A prospective randomized clinical trial to investigate the effect of silicone gel sheeting (Cica-Care) on post-traumatic hypertrophic scar among the Chinese population. Burns. 2006;32(6):678–83.
Warriner R, Burrell R. Infection and the chronic wound: a focus on silver. Adv Skin Wound Care. 2005;18 Suppl 1:2–12.
Manaker GM, Mehlis SL, Kasprowicz S. Dressings. In Dermatology Third Edition. Bolognia JL, Jorizo JL, Schaffer JV. 145, 2365–2379. 2012 Elsevier Limited.
Vermeulen H, van Hattem JM, Storm-Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev. 2007;24(1):CD005486.
Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds. 2003;15:149.
Hansson C. The effects of cadexomer iodine paste in the treatment of venous leg ulcers compared with hydrocolloid dressing and paraffin gauze dressing, cadexomer iodine study group. Int J Dermatol. 1998;37:390–6.
Shevchenko RV, James SL, James SE. A review of tissue-engineered skin biocontstructs available for skin reconstruction. J R Soc Interface. 2010;7(43):229–58. This review summarizes the bio-engineered components and relevant data for skin substitutes.
Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33:405–13.
Epicel (cultured epithelial autografts) [package insert]. Cambridge, MA; Genzyme; Revised September, 2007.
Carsin H, Ainaud P, Le Bever H, Rives J, Lakhel A, Stephanazzi J, et al. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns. 2000;26:379–87.
Kym D, Yim H, Yang HT, Cho YS, Hur J, Chun W, Kim JH. The application f cultured epithelial autografts improves survival in burns. Wound Repair Regen. 2015 [epub ahead of print].
Shahrokhi S, Arno A, Jeschke MG. The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen. 2014;22(1):14–22. Review article summarizing the use of dermal substitutes in burns.
Clark RA, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018–29.
Debels H, Hamdi M, Abberton K, Morrison W. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open. 2015;3(1):e284. This recent review article categorizes and summarizes data for dermal substitutes.
Melman L, Jenkins ED, Hamilton NA, Bender LC, Brodt MD, Deeken CR, et al. Early bio-compatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia. 2011;15(2):157–64.
Healy CM, Boorman JG. Comparison of E-Z Derm and Jelonet dressings for partial skin thickness burns. Burns Incl Therm Inj. 1989;15:52–4.
Vanstraelen P. Comparison of calcium sodium alginate (KALTOSTAT) and porcine xenograft (E-Z DERM) in the healing of split-thickness skin graft donor sites. Burns. 1992;18:145–8.
Cassidy C, St Peter SD, Lacey S, et al. Biobrane versus DuoDerm for the treatment of intermediate thickness burns in children: a prospective, randomized trial. Burns. 2005;31:890–3.
Feldman DL, Rogers A, Karpinski RH. A prospective trial comparing biobrane DuoDerm and xeroform for skin graft donor sites. Surg Gynecol Obstet. 1991;173:15.
Yang JY, Tsai YC, Noordhoff MS. Clinical comparison of commercially available biobrane preparations. Burns. 1989;15:197–203.
Truong AN, Kowal-Vern A, Latenser BA, Wiley DE, Walter RJ. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J Burns Wounds. 2005;4:e4.
Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus Hyaloskin in the treatment of difficult-to-heal wounds of mixed arterial/venous aetiology. Int Wound J. 2007;4(1):3–7.
Gordley K, Cole P, Hicks J, et al. A comparative, long-term assessment soft tissue substitutes: AlloDerm, Enduragen, and Dermamatrix. J Plast Recostr Aesthet Surg. 2009;62:849–50.
Insausti CL, Alcaraz A, García-Vizcaíno EM, Mrowiec A, López-Martínez MC, Blanquer M, et al. Amniotic membrane induces epithelialization in massive posttraumatic wounds. Wound Repair Regen. 2010;18:368–77.
Neox 100 (cryopreserved Human Amniotic Membrane) [package insert]. Doral, FL; Amniox Medical.
Gravante G, Delogu D, Giordan N, Morano G, Monone A, Esposito G. The use of hyalomatrix PA in the treatment of deep partial-thickness burns. J Burn Care Res. 2007;28:269–74.
Kumar RJ, Kimble RM, Boots R, et al. Treatment of partial-thickness burns: a prospective, randomized trial using TransCyte. ANZ J Surg. 2004;74:622–65.
Purdue FG, Hunt JL, Still Jr JM, et al. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft TC compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997;18(1, Part 1):52–7.
Purdue GF. Dermagraft-TC pivotal safety and efficacy study. J Burn Care Rehabil. 1997;18:S13–4.
Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701–5.
Boyd M, Flasza M, Johnson PA, et al. Integration and persistence of an investigational human living skin equivalent (ICX-SCN) in human surgical wounds. Regen Med. 2007;2:363–70.
Stark HJ, Boehnke K, Mirancea N, Willhauck MJ, Pavesio A, Fusenig NE, et al. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. J Investig Dermatol Symp Proc. 2006;11:93–105.
Uccioli L, TissueTech Autograph System Italian Study Group. A clinical investigation on the characteristics and outcomes of treating chronic lower extremity wounds using the tissuetech autograft system. Int J Low Extrem Wounds. 2003;2:140–51.
Apligraf [package insert]. Canton, MA; Organogenesis; Revised Dec 2010.
Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7:201–7.
Veves A, Falanga V, Armstrong DG, Apligraf Diabetic Foot Ulcer Study, et al. Graftskin, a human equivalent, is effective in the management of noninfected neuropathic foot ulcers: a prospective randomized multi-center clinical trial. Diabetes Care. 2001;24:290–5.
Griffiths M, Ojeh N, Livingstone R, Price R, Navsaria H. Survival of Apligraf in acute human wounds. Tissue Eng. 2004;10:1180–95.
OrCel™ (Bilayered Cellular Matrix) [package insert]. New York, NY; Ortec International, Inc; Revised Sept 2001.
Still J, Glat P, Silverstein P, Griswold J, Mozingo D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. 2003;29:837–41.
Oryan A, Zaker SR. Effects of topical application of honey on cutaneous wound healing in rabbits. J Veterinary Med Ser A. 1998;45(3):181–3.
Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2008; undefined: Rev 20
Rhoads DD, Wolcott RD, Kuskowski MA, et al.: Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 2009; 18: pp. 237–23808; un.
Frenkel O, Shani E, Ben-Bassat I, et al. Activated macrophages for treating skin ulceration: gene expression in human monocytes after hypo-osmotic shock. Clin Exp Immunol. 2002;128:59–66.
Hong L, Peptan IA, Colpan A, et al. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006;183:133–40.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Emily C. Newsom, Karen L. Connolly, and Kishwer S. Nehal declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Medical Surgery
Rights and permissions
About this article
Cite this article
Newsom, E.C., Connolly, K.L. & Nehal, K.S. Facilitating Healing of Granulating Wounds: Dressings, Dermal Substitutes, and Other Methods. Curr Derm Rep 4, 125–133 (2015). https://doi.org/10.1007/s13671-015-0108-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-015-0108-3
Keywords
- Dressings
- Dermal substitutes
- Skin substitutes
- Dermal matrices
- Dermal matrix
- Tissue-engineered skin substitute
- Wound care
- Surgical wounds
- Mohs surgery
- Skin equivalent
- Scaffold
- Bioengineered skin substitute
- Cultured epithelial autograft
- Allogenic fibroblasts
- Bioactive material
- Soft tissue substitute
- Wound healing
- Dermatology
- Dermatologic surgery
- Granulating wounds
- Secondary intention
- Second intent
- Skin cancer surgery