Abstract
Purpose of Review
The landscape of the coronavirus disease 2019 (COVID-19) pandemic has rapidly changed over the past 3 years. Paralleling this evolution, the scientific and medical communities have reported many novel findings relating to the infection’s epidemiology, transmission, diagnosis, and treatment. We review pertinent studies of COVID-19 therapeutics with an emphasis on their application to lung transplant recipients.
Recent Findings
Agents that have been well-studied for treating COVID-19 include antivirals (remdesivir, nirmatrelvir/ritonavir, molnupiravir), monoclonal antibodies, and immunomodulators (for example, corticosteroids and tocilizumab).
Summary
Remdesivir remains an essential therapy for managing mild-moderate COVID-19. Though highly efficacious for mild-moderate COVID-19 for outpatient therapy, ritonavir-boosted nirmatrelvir has limited use in lung transplant recipients due to significant drug-drug interactions. Monoclonal antibodies, though useful, are the most affected by the emergence of new viral variants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, the coronavirus disease 2019 (COVID-19) pandemic has resulted in over 529 million confirmed cases and over 6 million deaths worldwide [1]. While early studies conflicted about whether solid-organ transplant recipients (SOTRs) were at increased risk for severe disease [2, 3], more recently, data show that these immunocompromised patients have overall mortality exceeding eight times that of the general population [4]. This may be due to an impaired immunological response, with delayed development of IgG and cytokine-producing T cells early in the course of disease, compared to immunocompetent controls [5].
Among SOTRs, the risk of severe disease and poor outcomes seems highest among lung transplant recipients (LTRs). The mortality for LTRs with COVID-19 ranges from 17 to 25%, higher than that of non-lung SOTRs [4, 6, 7]. LTRs who survive COVID-19, especially moderate or severe disease, are at risk for chronic allograft dysfunction as evidenced by persistent spirometric abnormalities, including decreased exercise capacity, total lung capacity, and diffusing capacity [8, 9].
Promisingly, the mortality associated with COVID-19 is decreasing as the pandemic progresses. Heldman and colleagues compared outcomes of SOTRs in early 2020 to those in late 2020 and found a significant decline in hospitalizations and mortality; they relate this finding in part to the increasing use of remdesivir, corticosteroids, and convalescent plasma, as well as decreasing use of anti-interleukin-6 agents and hydroxychloroquine [10]. In addition to changes in COVID-19-directed therapeutics, the improved outcomes may also be associated with improvements in critical care practices and more widespread vaccination, which have similarly been observed in the general population [11,12,13]. However, LTRs may be less likely to mount a protective immunologic response to vaccination [14, 15], making COVID-19-directed therapies particularly important in this patient population.
This review will provide an overview of the COVID-19-directed therapeutics that are currently available with a focus on the evidence in LTRs, where available. This includes antiviral therapies, antibody therapies, immunomodulatory agents, and anticoagulation. We will also briefly discuss therapies that were used earlier in the pandemic but are no longer recommended based on current evidence. Readers should note that the evidence for COVID-19-directed therapeutics is a constantly evolving field, as the emergence of new SARS-CoV-2 variants may lead to the development of new treatments and/or render previously beneficial therapies less effective. Finally, although preventative measures like vaccination, masking, hand hygiene, and, until recently, tixagevimab/cilgavimab (Evusheld®) are important methods of preventing infection in LTRs, they are outside of the scope of this review.
Antiviral Medications
Remdesivir
To date, no trials have specifically studied the use of COVID-19 antivirals in LTRs. In May 2020, the first SARS-CoV-2-specific antiviral to be authorized under emergency use by the Food and Drug Administration (FDA) was remdesivir. This intravenous medication is an adenosine nucleotide prodrug that, following triphosphorylation within the host cell, is incorporated into the viral genome via the viral RNA-dependent RNA polymerase and causes chain termination. The ACTT-1 study compared outcomes in hospitalized patients with severe COVID-19 (pulmonary infiltrates, hypoxemia, oxygen supplementation, mechanical ventilation, or extracorporeal membrane oxygenation [ECMO]) who received up to 10 days of remdesivir to those who received placebo [16]. Authors demonstrated that remdesivir was associated with a significantly shorter time to recovery (10 days versus 15 days) and 50% increased odds of improvement by 2 weeks; however, there was no difference in all-cause mortality at 1 month. The recovery benefits were most significant to patients presenting within 10 days of symptom onset and requiring supplemental oxygen–but not mechanical ventilation or ECMO. Subsequently, two large open-label trials comparing 10 days of remdesivir to the standard of care demonstrated conflicting data. The DisCoVeRy trial found no improvement in 15-day clinical status or mortality [17]. The World Health Organization (WHO) Solidarity Trial failed to show a significant benefit of remdesivir over the standard of care in preventing in-hospital mortality or progression to needing mechanical ventilation [18•]. Duration of remdesivir therapy has also been a focus of study, with several studies comparing 10 days to 5 days [19, 20]. Both open-label studies demonstrated that a 5-day course of remdesivir, compared to a 10-day course, for patients admitted with moderate-to-severe COVID-19 resulted in similar rates of recovery by 11 days and similar times to improvement and recovery.
Although data are conflicting, in October 2020, remdesivir was fully FDA-approved for the treatment of all adults and children hospitalized with confirmed or suspected COVID-19. However, based on available evidence, current guidelines recommend remdesivir’s use, in addition to corticosteroids, specifically in patients hospitalized with COVID-19 within 10 days of symptom onset and requiring supplementary oxygen with the goal of speeding time to recovery and preventing the need for mechanical ventilation.
The most important limitation to using remdesivir for inpatients is the narrow window during the disease course when patients are expected to benefit. Remdesivir is not recommended for patients with severe renal dysfunction due to the concern of accumulation of the sulfobutylether β-cyclodextrin excipient, though several studies have shown that adverse outcomes were not significantly higher in patients with creatinine clearance < 30 mL/min or on dialysis, and therefore, remdesivir should still be considered in these patients [21,22,23]. Due to hepatotoxicity in early animal studies, remdesivir is not recommended in patients that have or subsequently develop elevated liver enzyme; however, in human trials, rates of elevated liver enzymes between those patients that get remdesivir and those that do not are comparable [17, 20].
Following a year of inpatient experience, the PINETREE trial studied the use of a 3-day course of remdesivir for outpatients with COVID-19 [24]. In this study, non-hospitalized, unvaccinated adults and adolescents with mild-to-moderate COVID-19 and risk for severe COVID were randomized to receive remdesivir or placebo within 7 days of their symptom onset. The authors concluded that a 3-day course of remdesivir led to an 87% relative reduction in all-cause mortality and COVID-19-related hospitalizations at 28 days. In this study, about 5% of subjects were considered immunocompromised, which did include SOTRs.
Oral Antivirals
To date, the two oral antivirals with the most supportive evidence for their use are nirmatrelvir and molnupiravir. Both have been studied and are recommended for outpatients with mild-to-moderate COVID-19 and risk factors for severe disease.
Nirmatrelvir is an oral antiviral agent which inhibits the SARS-CoV-2 3-chymotrypsin-like protease enzyme, also known as main protease (Mpro). This enzyme is essential for SARS-CoV-2 viral replication. Nirmatrelvir is co-administered with the HIV-1 protease inhibitor and potent CYP3A4 inhibitor, ritonavir, thereby increasing its half-life and serum concentrations [25]. The use of nirmatrelvir/ritonavir (Paxlovid®) for the outpatient management of COVID-19 was demonstrated in the EPIC-HR trial [26]. In this international randomized trial, non-hospitalized adults that received a 5-day course of nirmatrelvir/ritonavir within 5 days of symptoms onset had 89% relative risk reduction of all-cause mortality or COVID-19-related hospitalization at 28 days compared to those that received placebo. Included patients were unvaccinated against SARS-CoV-2 and had to have a risk factor for disease progression to severe COVID-19. Although solid-organ transplant was included as a risk factor for disease progression, only 12 subjects were considered immunosuppressed based on their underlying condition or medication, and the number of these that SOTRs was not published.
An important consideration for this therapy’s use in LTRs is the interactions with many other medications given ritonavir’s potent CYP3A4 inhibition. In patients on calcineurin inhibitors (CNIs), such as tacrolimus or cyclosporine, even a short course of this antiviral therapy has been associated with toxic increases in CNIs levels. This can result in nephrotoxicity, neurotoxicity, and the need to hold immunosuppressive therapy, which can potentiate rejection in an otherwise pro-inflammatory disease [27, 28]. For these reasons, the use of nirmatrelvir/ritonavir is not recommended in SOTRs on a CNI without the ability to closely monitor for drug levels and toxicities. An extensive list of drug-drug interactions and strategies to mitigate risks are published elsewhere [28•].
Precursors of molnupiravir were previously in development as oral antivirals against RNA viruses, particularly equine encephalitis viruses and chikungunya virus [29]; in 2018, molnupiravir was studied and demonstrated activity against influenza and respiratory syncytial virus in mouse models [29]. With the onset of the COVID-19 pandemic, molnupiravir was retested and marketed as a potential candidate for oral SARS-CoV-2 therapy. Molnupiravir is a prodrug of the molecule N-hydroxycytidine, which, following absorption and triphosphorylation, becomes incorporated into the viral genome by the virus’s RNA-dependent RNA polymerase and yields replicative errors and subsequent viral demise. In the MOVe-OUT study, 1433 non-hospitalized adults with symptomatic COVID-19 were randomized to a 5-day course of molnupiravir or placebo [30]. The use of molnupiravir was associated with a statistically significant reduction of 30% in 28-day all-cause mortality or COVID-19-associated hospitalization. Like the EPIC-HR, patients were unvaccinated, within 5 days of symptom onset, and at risk for progression to severe disease. Although no subjects were reportedly SOTRs, 4% of subjects did have chronic obstructive pulmonary disease.
Although its effects seem less than nirmatrelvir/ritonavir, molnupiravir is advantageous as it has minimal drug interactions, making it a more attractive option for SOTRs. Given its mechanism of action of inducing genomic mutations, there is a theoretical concern that it can cause embryotoxicity; therefore, its use is not recommended in pregnant women.
Studies like those for nirmatrelvir/ritonavir and molnupiravir were conducted in late 2020 and throughout 2021. Since, circulating viral variants changed and may not reflect the status of future variants. In the MOVe-OUT study, 32% of subjects were infected with the Delta variant and 11% with the Mu variant. Since December 2021, these variants have been primarily overtaken but Omicron subvariants; however, the most common mutations leading to the genesis of new variants occur in the viral spike protein, which is not a target of these antivirals, and activity should be preserved. Dosing and monitoring parameters for remdesivir, nirmatrelvir/ritonavir, and molnupiravir are summarized in Table 1.
The selective-serotonin reuptake inhibitor, fluvoxamine, was also studied as an oral option for the prevention of disease progression in high-risk individuals. This medication is widely available globally and well tolerated, which made it an attractive option for study. Its proposed benefit in COVID-19 relates to fluvoxamine’s potent agonism of the sigma-1 receptor; this transmembrane endoplasmic reticulum protein is found throughout most human tissues and is implicated in dampening inflammatory cytokine release and mass cell degranulation, among other physiological processes [31]. Fluvoxamine also has potential direct antiviral activity through inhibition of lysosomal release of coronavirus particles from infected cells; this latter mechanism has been proposed for other repurposed medications studied against COVID-19, such as azithromycin and non-steroid anti-inflammatory drugs [32]. The primary study evaluating fluvoxamine’s use for COVID-19 is the multicenter Brazilian TOGETHER trial, which randomized outpatients with COVID-19 and high risk of progression to either receive a 10-day course of fluvoxamine or placebo [33••]. The trial demonstrated a 32% risk reduction in a composite outcome of COVID-19-related emergency room visits and hospitalization in 28 days. However, in the secondary analyses, only emergency room visits were significantly decreased, without any significant impact on hospitalization, death, or need for mechanical ventilation.
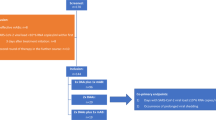
Proposed strategies for outpatient and inpatient management of LTRs with COVID-19 are highlighted in Figs. 1 and 2.
Proposed outpatient management strategy for lung transplant recipients with COVID-19. †Due to inferior efficacy, molnupiravir is not a preferred choice and should only be used if remdesivir or nirmatrelvir/ritonavir are not possible options (e.g., drug-drug interactions, toxicities, or unavailability)
Proposed inpatient management strategy for lung transplant recipients with COVID-19. Abbreviations: CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannulae; LTR, lung transplant recipient; MV, mechanical ventilation; NIV, non-invasive ventilation. †Although specific CRP thresholds are not specified in the guidelines of the National Institute of Health [93], 75–100 mg/L were used in the REMAP-CAP and RECOVERY trials [63, 64]. ‡Baricitinib should be discontinued if the patient is discharged before 14 days. §If tocilizumab and baricitinib are not available, they may be substituted with either tofacitinib or sarilumab
Monoclonal Antibody Therapies
Monoclonal antibody (mAb) therapy targeting the receptor-binding domain of the SARS-CoV-2 spike protein has been a mainstay of therapy for high-risk patients presenting with mild-to-moderate COVID-19 not requiring hospitalization [34••, 35]. Clinical trial data have shown mAb therapy to be safe and associated with a significant reduction (70–87%) in need for hospitalization or death among those with high-risk medical conditions [36,37,38]. Several different mAb therapy options have been available over the course of the COVID-19 pandemic–bamlanivimab, bamlanivimab-etesevimab, casirivimab-imdevimab, sotrovimab, and bebtelovimab. With the ongoing emergence of new viral variants, the neutralizing activity of mAb therapy wanes or is lost, limiting the duration in which any one mAb will remain a therapeutic option. Until recently, bebtelovimab was the only option for the predominant Omicron variant circulating; however, it no longer has retained activity since late 2022 [39].
Most data for the solid-organ transplant patient population are derived from retrospective observational studies [40,41,42,43,44,45]. Of the studies that included any SOTRs, administration of mAb therapy within 3–7 days of symptom onset was associated with only 0–22.2% of patients requiring emergency department care or hospital admission, and only one observed death. In a single-center cohort, hospitalization and 90-day mortality were otherwise as high as 66% and 34%, respectively, among 32 LTRs [7]; only 21% of the patients in the cohort received mAb therapy as outpatients, and only two patients would go on to develop severe COVID-19 after receiving mAb. Based on these data, it appears that mAb administration effectively reduces hospitalizations and death in LTRs. Other studies are similarly promising. In one cohort of 133 LTRs admitted with COVID-19, 31 patients received mAb therapy within 5 days of symptom onset, with only one progressing to severe disease [45]. In this retrospective analysis, mAb use was independently associated with in-hospital survival. Only one of the available studies had a comparator group of SOTRs that did not receive mAb therapy. Sarrell et al. evaluated the early use of mAb therapy in solid-organ transplant patients (N = 93, including 11 lung transplant patients), with 19 patients receiving mAb therapy [44]. Among those that received mAb therapy 30-day hospitalization rate was 8.7% compared to 15.3% among those that did not receive a mAb. In all the available studies evaluating mAb use in solid-organ transplant patients, mAb therapy was well tolerated, with rarely reported mild infusion-related reactions.
When an active mAb therapy is available, its role in outpatient management is essential for lung transplant recipients. The main challenge with this therapy is its availability only as an intravenous treatment option and the loss of neutralizing activity against new and emerging SARS-CoV-2 variants. On the other hand, it does not have the significant drug interaction as nirmatrelvir/ritonavir and requires only one intravenous administration. Studies are now evaluating new mAbs that may be active against the newest variants [46, 47•].
In addition to mAb therapy used to treat active disease, there is also a mAb indicated for pre-exposure prophylaxis among high-risk patients, tixagevimab/imdevimab. In the PROVENT trial, when administered to high-risk patients that have not recently been exposed and that do not have active disease, use was associated with a 77% reduction in infection risk compared to placebo [48]. Specifically, in the solid-organ transplant population, one study evaluating the 60-day incidence of breakthrough infection found a 1.8% incidence among those that received tixagevimab/imdevimab (n = 222) compared to a 4.7% incidence among those that did not (n = 222) [49]. As of November 2022, due to the prevalence of resistant variants, tixagevimab/imdevimab is unlikely to be effective [50].
Immunomodulatory Therapies
Corticosteroids
Of the available therapies for COVID-19, dexamethasone has consistently proved to provide a mortality benefit among those with severe disease requiring oxygen supplementation or mechanical ventilation. The clinical benefit of dexamethasone for patients with COVID-19 was first identified in the RECOVERY trial, where a daily dose of 6 mg for 10 days was associated with statistically significant reductions in death by 12% and 3% among mechanically ventilated patients and those requiring oxygen supplementation, respectively [51]. Since, several studies have confirmed these findings, making dexamethasone a critical component of COVID-19 treatment in patients with severe disease [52]. While data specific to LTRs are lacking, the reported benefits of dexamethasone are likely applicable to these patients, given that LTRs can often present with a more severe hyperinflammatory presentation of COVID-19 [53, 54]. Additional studies have shown that higher dexamethasone doses may be considered in patients with more severe disease. For example, the STEROID 2 trial compared outcomes between those receiving 6 mg daily to those receiving 12 mg daily for 10 days, and while there was no significant difference in the primary outcome of days free of life support within the first month of diagnosis, Bayesian analysis revealed that the higher dose might provide additional benefit among those requiring a high level of respiratory support [55]. Another open-label study comparing the efficacy of high-dose (20 mg daily for 5 days followed by 10 mg daily for 5 days) to low-dose (6 mg daily) dexamethasone in patients with severe COVID-19 concluded that the higher dose was associated with significantly reduced risk of progression from 31.4 to 16.3%. However, there was no significant difference in 28-day mortality, time to recovery, or clinical status at various time points between the two dosing strategies [56]. Guidelines preferentially recommend a daily dose of 6 mg of dexamethasone (or equivalent dose of another corticosteroid) for 10 days or until hospital discharge [34••].
Janus Kinase (JAK) Inhibitors
Baricitinib is now recommended for use in patients with severe COVID-19 based on the findings of the ACTT-2 and COV-BARRIER clinical trials. The ACTT-2 trial, comparing baricitinib with standard of care to standard of care alone, found the use of baricitinib to be associated with reduced time to recovery when given in combination with remdesivir in hospitalized patients requiring oxygen supplementation without mechanical ventilation [57]. In this trial, corticosteroids were not included in the standard of care regimens. In the COV-BARRIER trial, baricitinib with the standard of care was compared to standard of care alone in patients having at least one elevated inflammatory marker and not requiring mechanical ventilation [58]. Most patients in the trial received dexamethasone, while only 18% of patients were also receiving remdesivir. The addition of baricitinib saw a 38.2% relative reduction in 28-day all-cause mortality.
Tofacitinib, another JAK inhibitor, was evaluated in the STOP-COVID trial and, compared to placebo, was found to be associated with a significant reduction in the cumulative incidence of death or respiratory failure at day 28 from 29 to 18.1% [59]. Notably, while 78.5% of patients received concomitant corticosteroid therapy, no patients received remdesivir. Importantly, we do not have clinical trial data definitively showing benefit in patients also receiving remdesivir with dexamethasone in combination with a JAK inhibitor. Guidelines recommend using baricitinib (tofacitinib as an alternative) in patients requiring oxygen supplementation if presenting with rapidly increasing oxygen needs and systemic inflammation, in addition to dexamethasone and remdesivir. Baricitinib is dosed at 4 mg orally once daily for 14 days or until hospital discharge; this agent requires renal dose adjustment. Data specific to lung transplant patients with COVID-19 for the use of JAK inhibitors are not available; furthermore, the implications of using JAK inhibitors in patients who are also receiving dexamethasone and other immunosuppressive therapies in terms of efficacy and adverse effects (e.g., increased risk of infection) are unknown at this time. A recent FDA warning was also released regarding an increased risk of serious heart-related complications (myocardial infarction, stroke, blood clots, cancer, death) with the use of JAK inhibitors among those receiving therapy for arthritis and ulcerative colitis [60]. While unclear if this risk exists with the use of JAK inhibitors for COVID-19 where shorter courses are implemented, this risk should be considered with use given the association with thrombosis and other cardiac complications with COVID-19 itself [61].
Anti-interleukin-6 (IL-6) Receptor Monoclonal Antibody
Tocilizumab has been found in some clinical trials to be associated with a reduced need for mechanical ventilation and mortality in patients with severe COVID-19, particularly among patients presenting with evidence of a hyperinflammatory response [62,63,64]. However, data regarding the clinical benefit of tocilizumab have been mixed, with earlier studies (where few patients were receiving concomitant corticosteroids) failing to identify a mortality benefit with the use of tocilizumab in patients with severe COVID-19 [65, 66]. In the more recent trials where over 80% of patients also received corticosteroids, the use of tocilizumab was associated with several improved clinical outcomes. The REMAP-CAP trial, which enrolled patients within 24 h of starting organ support in the ICU, found a significant improvement in the number of organ support-free days (OR 1.64 [95% CI, 1.17–2.91]) and increased survival at 90 days (OR 1.61 [95% CI, 1.25–2.08]) [64]. The second major trial to identify a clinical benefit of tocilizumab was the RECOVERY trial, which enrolled patients with hypoxia and evidence of systemic inflammation (C-reactive protein ≥ 75 mg/L), found a significant reduction in mortality at 28 days (31% versus 35% placebo, RR 0.85 [95% CI, 0.76–0.94]) [63]. Patients in the RECOVERY trial that received tocilizumab were also more likely to be discharged within 28 days (57% versus 50% placebo, rate ratio 1.22 [95% CI, 1.12–1.22]), and for those not requiring mechanical ventilation at baseline, patients receiving tocilizumab were also less likely to progress to requiring mechanical ventilation or death (35% versus 42% placebo, rate ratio 0.84 [95% CI, 0.77–0.92]).
Guidance from the National Institute of Health (NIH) and Infectious Diseases Society of America (IDSA) recommends the consideration of tocilizumab (or sarilumab as an alternative IL-6 monoclonal antibody if tocilizumab is not available) in combination with dexamethasone in patients requiring oxygen supplementation, mechanical ventilation, or ECMO presenting with rapidly increasing oxygen needs and systemic inflammation. The recommended dosing is 8 mg/kg (based on actual body weight) as a single dose. While additional doses could be administered at the treating clinician’s discretion in clinical trials, there is insufficient evidence to support recommending a second dose. However, caution is advised by the NIH in terms of the use of tocilizumab not adequately represented in clinical trials, including immunocompromised hosts. There are reports, mainly from observational studies, of increased rates of secondary infections developing following administration of tocilizumab, including invasive fungal infections, strongyloidiasis, and severe bacterial infections [67,68,69,70]. This risk may be a more significant concern in patients already immunosuppressed at baseline.
Available data from the solid-organ transplant population are also primarily based on observational studies for this therapeutic option as well. Pereira et al. evaluated the safety and efficacy of tocilizumab among SOTRs in a matched cohort study including 58 patients (15 patients included were LTRs) [71]. Compared to the matched control group, while outcomes were not statistically different, mortality was higher (41% versus 28%, p = 0.27), the hospital discharge rate was lower (52% versus 72%, p = 0.26), and the occurrence of secondary infections was higher (34% versus 24%, p = 0.55) in the tocilizumab group. Another study, which included 46 SOTRs (only one of these patients was an LTR) in two transplant centers in Saudi Arabia, compared outcomes among those that received tocilizumab (n = 21) versus those that received standard of care alone (n = 25) [72]. No significant difference in mortality (14.3% versus 4%, p = 0.318) or mechanical ventilation requirements was observed (23.8% versus 24%, p = 0.711); however, those receiving tocilizumab had a significantly shorter length of stay (9.6 days versus 20.7 days, p > 0.001). There was no difference in the rate of secondary infections between groups in this analysis.
The role of tocilizumab in the management of severe and rapidly progressing COVID-19 in lung transplant patients is unclear, given the sparsity of data. In small observational studies, no mortality benefit was observed. With the potential for harm from the risk of secondary infections in this patient population already at high risk for invasive fungal and bacterial infections, it is not evident that the potential benefits outweigh the potential risks.
Anti-IL-1 Receptor Antibody
Anakinra is a recombinant, nonglycosylated form of human IL-1 receptor antagonist. In the double-blind, randomized, placebo-controlled SAVE-MORE trial, the early administration of anakinra was evaluated in hospitalized patients with moderate-to-severe COVID-19 pneumonia who required oxygen and had an elevated plasma soluble urokinase plasminogen activator receptor (suPAR) level [73]. Compared to those receiving placebo, those treated with anakinra saw statistically significantly lower 1-month mortality (3.2% vs. 6.9%) and decreased length of hospitalization (7.8 days vs. 8.5 days). Although this therapy was granted a EUA, its use is limited by the sparse availability of the suPAR assay outside of clinical trials. These trials of antivirals and immunomodulators are summarized in Table 2.
Convalescent Plasma
High-titer convalescent plasma (HTCP) is a pooled blood product of patients who have survived a prior SARS-CoV-2 infection and has been studied for treatment in those with a new infection. HTCP has not been shown to play a role in treating immunocompetent outpatient or hospitalized adults with SARS-CoV-2 infection [74,75,76]. In immunocompromised patients, such as those with hematologic malignancies or SOTRs, who cannot mount an adequate humoral response after vaccination, HTCP may serve a role, particularly early in the disease course, to clear the SARS-CoV-2 virus [77]. Because of the emergence of new variants throughout the course of the global pandemic, HTCP that was collected before any currently predominant variant may not be beneficial for administration in immunocompromised hosts needing this humoral support [78]. Due to conflicting data, HTCP has largely been replaced by the monoclonal therapies previously mentioned.
Non-recommended Therapies
At the onset of the pandemic, the immediate need for outbreak management led to the study of many therapies with a theoretical basis for treating COVID-19. As the aforementioned targeted antivirals and immunomodulatory therapies were shown to be both efficacious and safe, what were once popular agents have now been either discredited, found to be harmful, or lost activities against newer SARS-CoV-2 variants.
Almost a dozen randomized trials and observational studies of the antimalarials, hydroxychloroquine and chloroquine, with or without azithromycin, failed to prove these drugs’ efficacy for hospitalized or non-hospitalized patients with COVID-19 [18•, 79,80,81]. Repeatedly, there has been no significant improvement in mortality, time to recovery, or reduction in hospitalization or disease progression associated with either drug. Furthermore, their use specifically for COVID-19 was associated with higher rates of adverse effects [80, 81].
The anti-parasitic medication, ivermectin, is another therapy that was attempted to be repurposed for SARS-CoV-2 treatment in the early stages of the COVID-19 pandemic. The proposed mechanisms of action include inhibition of host nuclear transport activity and interference with spike protein binding to the host cell membrane [82]. Despite these promising in vitro data, concentrations needed to achieve antiviral efficacy in vivo exceed over 100 times those considered safe in humans [83]. To date, no high-quality clinical trial data support using ivermectin for COVID-19 treatment or prophylaxis [84,85,86].
Additional emerging therapies being investigated are interferon therapy [18•, 87], granulocyte-macrophage colony-stimulating factor inhibitors [88, 89], and mesenchymal stem cell therapy [90, 91]; however, currently, no high-quality evidence exists to support their use outside of clinical trials.
Discussion
The initial strategies for treating COVID-19 have mainly focused on inhibiting viral entry into host proteins in the initial phase of infection and immunomodulating the host immune response in the later stage of infection. With extensive global cooperation to methodically study a broad range of therapies, alongside sharing real-time clinical experiences, we are continually learning about the SARS-CoV-2 virus and the variable immune responses between different populations. We have come to understand better how the timing of initiating therapy and the host’s underlying disease state impact the patient outcomes and, in the case of lung transplantation, allograft function. Our knowledge will never be complete due to the everchanging SARS-CoV-2 virus and the global landscape. Not only will new therapies be required as the virus evolves, but so too novel methods of administration of currently available therapies will be instrumental in facilitating timely outpatient treatment when these therapies are likely to have the greatest success. Studies to evaluate potential synergy or combinations among treatment modalities may also help find ways of targeting the virus while mitigating the risk of developing resistance (a major global challenge that is in part due to prolonged therapies in immunocompromised hosts unable to clear the virus).
As with much of modern medicine, prevention is often the best treatment. We have seen that vaccinations are likely the most effective way to prevent COVID-19 in the general population; however, because of the high degree of immunosuppression limiting the humoral immune response and lungs’ constant exposure to environmental pathogens, vaccination alone may not suffice in LTRs.
The COVID-19 therapy pipeline continues to be robust as more studies are conducted to repurpose current drugs and discover new ones to target either viral or host proteins to decrease SARS-CoV-2 infectivity, with over 700 drug development programs currently in the planning stages and over 450 trials having been reviewed by the FDA in the USA alone [92]. Future developments must focus on therapies that target more conserved viral structures, which may be less susceptible to mutations as new variants emerge. This strategy will too prove helpful for future emerging viral pandemics.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization. Weekly epidemiological update on COVID-19 - 15 June 2022. 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---15-june-2022. Accessed 15 Jun 2022.
Pereira MR, Arcasoy S, Farr MA, Mohan S, Emond JC, Tsapepas DS, et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23:e13637.
Rinaldi M, Bartoletti M, Bussini L, Pancaldi L, Pascale R, Comai G, et al. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis. 2021;23:e13421.
An W, Wang Q, Kim T-E, Kang J-S. Clinical characteristics and outcome of coronavirus disease 2019 infection in patients with solid organ transplants: a systematic review and meta-analysis. J Infect Public Health. 2022;15:365–72.
Favà A, Donadeu L, Sabé N, Pernin V, González-Costello J, Lladó L, et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21:2749–61.
Ochman M, Latos M, Galle D, Niepokój K, Stącel T, Urlik M, et al. COVID-19 among lung transplant recipients: a single center study. Transplant Proc. 2022;54(4):913–6.
Laothamatas K, Hum J, Benvenuto L, Shah L, Grewal HS, Pereira M, et al. One year into the pandemic: evolving COVID-19 outcomes in lung transplant recipients, a single-center experience. Transplant Direct. 2022;8:e1296.
Kamp JC, Hinrichs JB, Fuge J, Ewen R, Gottlieb J. COVID-19 in lung transplant recipients–risk prediction and outcomes. PLoS ONE. 2021;16:e0257807 Chen RJ, editor.
Magnusson JM, Larsson H, Alsaleh A, Ekelund J, Karason K, Schult A, et al. COVID-19 in lung transplant recipients: an overview of the Swedish national experience. Transpl Int. 2021;34:2597–608.
Heldman MR, Kates OS, Safa K, Kotton CN, Georgia SJ, Steinbrink JM, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am J Transplant. 2022;22:279–88.
Yeates EO, Nahmias J, Chinn J, Sullivan B, Stopenski S, Amin AN, et al. Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure. PLoS ONE. 2021;16:e0253767 Chen RJ, editor.
Horwitz LI, Jones SA, Cerfolio RJ, Francois F, Greco J, Rudy B, et al. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2021;16:90–2.
Suthar AB, Wang J, Seffren V, Wiegand RE, Griffing S, Zell E. Public health impact of covid-19 vaccines in the US: observational study. BMJ. 2022;377:e069317.
Havlin J, Skotnicova A, Dvorackova E, Hubacek P, Svorcova M, Lastovicka J, et al. Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein–specific T cells in lung transplant recipients. Transplantation. 2022;106:e183–4.
Callaghan CJ, Mumford L, Curtis RMK, Williams SV, Whitaker H, Andrews N, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106:436–46.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 – final report. N Engl J Med. 2020;383:1813–26.
Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2022;22:209–21.
• WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19 – Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497–511. Findings from this study demonstrate the lack of efficacy of hydroxychloroquine and lopinavir indecreasing in-hosital COVID-19 mortality.
Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827–37.
Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048.
Pettit NN, Pisano J, Nguyen CT, Lew AK, Hazra A, Sherer R, et al. Remdesivir use in the setting of severe renal impairment: a theoretical concern or real risk? Clin Infect Dis. 2021;73:e3990–5.
Ackley TW, McManus D, Topal JE, Cicali B, Shah S. A valid warning or clinical lore: an evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother. 2021;65:e02290-e2320.
Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, et al. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Neph. 2020;31:1384–6.
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–15.
U.S. Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for PaxlovidTM. 2022. https://www.fda.gov/media/155050/download. Accessed 7 Jun 2022.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–408.
Prikis M, Cameron A. Paxlovid (nirmatelvir/ritonavir) and tacrolimus drug-drug interaction in a kidney transplant patient with SARS-2-CoV infection: a case report. Transpl Proc. 2022;54(6):1557–60.
• Infectious Diseases Society of America. Management of drug interactions with nirmatrelvir/ritonavir (Paxlovid®): resource for clinicians. 2022. https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/treatment/idsa-paxlovid-drug-interactions-resource-5-6-22-v1.1.pdf. Accessed 31 May 2022. Findings from this study demonstrate the lack of efficacy of hydroxychloroquine and lopinavir in decreasing in-hosital COVID-19 mortality.
Tian L, Pang Z, Li M, Lou F, An X, Zhu S, et al. Molnupiravir and its antiviral activity against COVID-19. Front Immunol. 2022;13:855496.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20.
Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021;12:652688.
Homolak J, Kodvanj I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int J Antimicrob Agents. 2020;56:106044.
•• Reis G, dos Santos Moreira-Silva EA, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42-51. National guidelines with most up-to-date evidence for COVID-19 therapies.
•• COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institute of Health. 2022. https://www.covid19treatmentguidelines.nih.gov/. Accessed 6 Apr 2022. National guidelines with most up-to-date evidence for COVID-19 therapies.
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC-C, et al. Infectious disease society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020;ciaa478.
Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Rodrigues Falci D, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236.
Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–92.
Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–51.
Iketani S, Liu L, Guo Y, Liu L, Chan JF-W, Huang Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–6.
Kutzler HL, Kuzaro HA, Serrano OK, Feingold A, Morgan G, Cheema F. Initial experience of bamlanivimab monotherapy use in solid organ transplant recipients. Transpl Infect Dis. 2021;23. https://onlinelibrary.wiley.com/doi/10.1111/tid.13662. Accessed 22 Jun 2022.
Dhand A, Lobo SA, Wolfe K, Feola N, Nabors C. Bamlanivimab for treatment of COVID‐19 in solid organ transplant recipients: early single‐center experience. Clin Transplant. 2021;35. https://onlinelibrary.wiley.com/doi/10.1111/ctr.14245. Accessed 22 Jun 2022.
Jan MY, Sayegh SE, Webb HT, Adebiyi O, Anderson MD, Mishler DP, et al. Bamlanivimab for mild to moderate COVID-19 in kidney transplant recipients. Kidney Int Rep. 2021;6:2468–71.
Yetmar ZA, Beam E, O’Horo JC, Ganesh R, Bierle DM, Brumble L, et al. Monoclonal antibody therapy for COVID-19 in solid organ transplant recipients. Open Forum Infect Dis. 2021;8:ofab255.
Sarrell BA, Bloch K, El Chediak A, Kumm K, Tracy K, Forbes RC, et al. Monoclonal antibody treatment for COVID‐19 in solid organ transplant recipients. Transplant Infectious Dis. 2022;24. https://onlinelibrary.wiley.com/doi/10.1111/tid.13759. Accessed 22 Jun 2022.
Gottlieb J, Kolditz M, Gade N, Welte T, Kneidinger N. Benefit of monoclonal antibodies in early treatment of COVID-19 after lung transplantation – a retrospective analysis in two centers. Eur Respir J. 2022;60:2200124.
National Library of Medicine (U.S.). A randomized, double-blind, placebo-controlled, escalating single dose, phase 1 & phase 2 study to evaluate the safety and efficacy of inhaled IBIO123 in participants with severe COVID-19 illness. 2022. https://clinicaltrials.gov/ct2/show/study/NCT05303376. Accessed 15 Jan 2023.
• Lee JY, Lee JY, Ko J-H, Hyun M, Kim HA, Cho S, et al. Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease. Front Immunol. 2021;12:772320. Findings of this study were the first to demonstrate a mortality benefit in using dexamethsone for patients hospitalized with COVID-19 who require supplemental oxygen or mechanical ventilation.
Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386(23):2188–200.
Al Jurdi A, Morena L, Cote M, Bethea E, Azzi J, Riella LV. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the Omicron wave. Am J Transplant. 2022;22(12):3130–6.
Wang Q, Li Z, Ho J, Guo Y, Yeh AY, Mohri H, et al. Resistance of SARS-CoV-2 omicron subvariant BA.4.6 to antibody neutralisation. Lancet Infect Dis. 2022;22:1666–8.
The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704.
The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330.
Mohanka MR, Mahan LD, Joerns J, Lawrence A, Bollineni S, Kaza V, et al. Clinical characteristics, management practices, and outcomes among lung transplant patients with COVID-19. J Heart Lung Transplant. 2021;40:936–47.
Saez-Giménez B, Berastegui C, Barrecheguren M, Revilla-López E, Los Arcos I, Alonso R, et al. COVID-19 in lung transplant recipients: a multicenter study. Am J Transplant. 2021;21:1816–24.
The COVID STEROID 2 Trial Group, Russell L, Uhre KR, Lindgaard ALS, Degn JF, Wetterslev M, et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326:1807.
Taboada M, Rodríguez N, Varela PM, Rodríguez MT, Abelleira R, González A, et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2021;60:2102518.
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807.
Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–18.
Guimarães PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406–15.
Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. 2021. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death. Accessed 2 Dec 2021.
Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–7.
Salama C, Kaplan-Lewis E, Durrance R, Wong L, Arumugam V, Fabbri M. Tocilizumab for severe and critical COVID-19 pneumonia in queens. NYC Infect Dis Clin Pract. 2021;29:e215–20.
Abani O, Abbas A, Abbas F, Abbas M, Abbasi S, Abbass H, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–45.
The REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–502.
Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:215–27.
Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–16.
Lier AJ, Tuan JJ, Davis MW, Paulson N, McManus D, Campbell S, et al. Case report: disseminated strongyloidiasis in a patient with COVID-19. Am J Trop Med Hyg. 2020;103:1590–2.
Marchese V, Crosato V, Gulletta M, Castelnuovo F, Cristini G, Matteelli A, et al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection. 2021;49:539–42.
Pettit NN, Nguyen CT, Mutlu GM, Wu D, Kimmig L, Pitrak D, et al. Late onset infectious complications and safety of tocilizumab in the management of COVID-19. J Med Virol. 2021;93:1459–64.
Sandhu G, Piraino ST, Piticaru J. Secondary infection risk in patients with severe COVID-19 pneumonia treated with tocilizumab. Am J Ther. 2022;29:e275–8.
Pereira MR, Aversa MM, Farr MA, Miko BA, Aaron JG, Mohan S, et al. Tocilizumab for severe COVID-19 in solid organ transplant recipients: a matched cohort study. Am J Transplant. 2020;20:3198–205.
Yamani AH, Alraddadi BM, Almaghrabi RS, Amer AA, Mehdawi FS, AL‐Hamzi MA, et al. Early use of tocilizumab in solid organ transplant recipients with COVID‐19: a retrospective cohort study in Saudi Arabia. Immun Inflam Dis. 2022;10. https://onlinelibrary.wiley.com/doi/10.1002/iid3.587. Accessed 23 Jun 2022.
Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–60.
Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med. 2021;385:1951–60.
Menichetti F, Popoli P, Puopolo M, Spila Alegiani S, Tiseo G, Bartoloni A, et al. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open. 2021;4:e2136246.
The RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049–59.
Focosi D, Franchini M. Potential use of convalescent plasma for SARS-CoV-2 prophylaxis and treatment in immunocompromised and vulnerable populations. Expert Rev Vaccines. 2022;21(7):877–84.
Wang B, Goh YS, Prince T, Ngoh EZX, Salleh SNM, Hor PX, et al. Resistance of SARS-CoV-2 variants to neutralization by convalescent plasma from early COVID-19 outbreak in Singapore. NPJ Vaccines. 2021;6:125.
The RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–40.
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383:2041–52.
Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial. Clin Infect Dis. 2021;73:e4073–81.
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787.
Chaccour C, Hammann F, Ramón-García S, Rabinovich NR. Ivermectin and COVID-19: keeping rigor in times of urgency. Am J Trop Med Hyg. 2020;102:1156–7.
Reis G, Silva EASM, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with ivermectin among patients with Covid-19. N Engl J Med. 2022;386:1721–31.
Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21:635.
Lim SCL, Hor CP, Tay KH, Mat Jelani A, Tan WH, Ker HB, et al. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med. 2022;182:426.
Feld JJ, Kandel C, Biondi MJ, Kozak RA, Zahoor MA, Lemieux C, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med. 2021;9:498–510.
Temesgen Z, Burger CD, Baker J, Polk C, Libertin CR, Kelley CF, et al. Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. Lancet Respir Med. 2022;10:237–46.
Xi A, Luo Y, Guan J-T, Wang W-J, Xu Z-H. Efficacy and safety of granulocyte–macrophage colony-stimulating factor (GM-CSF) antibodies in COVID-19 patients: a meta-analysis. Inflammopharmacol. 2022. https://link.springer.com/10.1007/s10787-022-01105-9. Accessed 16 Dec 2022.
Kirkham AM, Bailey AJM, Monaghan M, Shorr R, Lalu MM, Fergusson DA, et al. Updated living systematic review and meta-analysis of controlled trials of mesenchymal stromal cells to treat COVID-19: a framework for accelerated synthesis of trial evidence for rapid approval–FASTER approval. Stem Cells Transl Med. 2022;11:675–87.
Yao W, Dong H, Qi J, Zhang Y, Shi L. Safety and efficacy of mesenchymal stem cells in severe/critical patients with COVID-19: a systematic review and meta-analysis. eClinicalMedicine. 2022;51:101545.
Ledford H. A deluge of new drugs for COVID. Nature. 2022;603:25–7.
COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institute of Health. 2022. https://www.covid19treatmentguidelines.nih.gov/. Accessed 15 Jan 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
DZP Friedman declares that he has no conflicts of interest. N Pettit declares that she has no conflicts of interest. E Mackenzie declares that she has no conflicts of interest. J Pisano receives grant support from Pfizer, Moderna, and Gilead.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Friedman, D.Z.P., Pettit, N.N., MacKenzie, E. et al. Current and Emerging Therapies for COVID-19 in Lung Transplantation. Curr Pulmonol Rep 12, 23–35 (2023). https://doi.org/10.1007/s13665-023-00302-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-023-00302-3