Abstract
Purpose of Review
The aim of this paper is to present basic data on pleural manometry and to outline the advances in its use as both a research tool enabling a better understanding of pleural pathophysiology and as a clinical tool useful in management strategy planning in patients with pleural diseases. To discuss updates and current trends in the development of pleural manometry, a search of the literature on pleural manometry published in recent years was performed.
Recent Findings
The technique of pleural manometry has significantly evolved over the last 40 years from simple water manometers to electronic or digital devices which enable the measurement and recording of instantaneous pleural pressure. Although to date it is mainly used as a research tool, pleural manometry has the potential to be applied in clinical practice. Recent studies demonstrated that monitoring of pleural pressure changes during therapeutic thoracentesis does not seem to be helpful in predicting re-expansion pulmonary edema and procedure-related chest discomfort. On the other hand, measurement of pleural elastance plays an important role in the diagnosis of unexpandable lung in patients with malignant pleural effusion facilitating determination of the optimal management strategy. Additionally, it allows for study of newly discovered phenomena, including pleural pressure pulse assessment and the impact of continuous positive airway pressure and cough on pleural pressure.
Summary
Pleural manometry is an established technique of pleural pressure measurement. Despite recent advances, its role in clinical practice remains undetermined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pleural cavity consists of two serous membranes, the visceral pleura closely applied to the lung and the parietal pleural layer, lining the chest wall, with a narrow space between. This space contains a very small amount of pleural fluid (a thin layer of 0.02 μm–2.0 mm in width) which is continuously produced and resorbed [1•]. The pleural fluid reduces friction between both pleural membranes and allows the lung to slide against the chest wall during the respiratory cycle. A unique feature of the pleural cavity is the negative, sub-atmospheric, pressure within this space, ranging between −3 and −5 cmH2O at functional residual capacity (FRC) and −6 to −10 cmH2O during the inspiratory phase of quiet breathing [1•, 2]. If the elastic recoil of the pleura is neglected, the pleura itself is passive in terms of pleural pressure generation, with changes in pleural pressure (Ppl) depending exclusively on external forces affecting the parietal and visceral pleura, mainly the function of respiratory muscles and the elastic recoil forces of the lung and the chest wall [1•]. Alterations in Ppl are directly responsible for lung expansion and deflation during the respiratory cycle [1•, 2, 3]. It should be emphasized that under certain conditions, the range of Ppl changes is much wider than presented above with decreases to −100 cmH2O during forced inspiration against central airway obstruction (e.g., in patients with obstructive sleep apnoea) [4] and increases to 400 cmH2O during forceful coughing maneuvers [5].
Although the salience of pleural physiological measurements remains debated, with doubts supported by the absence of the pleural cavity in some large mammals (e.g., elephants) and observations that ventilation and pulmonary gas exchange remain intact after pleurodesis [6,7,8], afflictions influencing pleural volume and increasing Ppl unquestionably produce symptoms and impact on the lung function of patients with common pleural pathologies, i.e., pleural effusion and pneumothorax.
Pleural effusion affects nearly 1.5 million patients per year in the USA [9] with approximately 150,000 diagnostic or therapeutic thoracenteses performed annually [10•, 11]. According to one large recent study, the incidence of spontaneous pneumothorax seems to be higher compared to that reported previously, with an admission-based estimated prevalence for primary spontaneous pneumothorax of 8.2 (95% CI, 7.8–8.6) and 2.5 (95% CI, 2.3–2.7) per 100,000 population in male and female, respectively, and for secondary spontaneous pneumothorax of 12.0 (95% CI, 11.6–12.5) and 4.5 (95% CI, 4.2–4.7) per 100,000 population in male and female, respectively [12•].
The measurement of Ppl and the pattern of changes over time in patients with pleural disease may help to optimize diagnosis and guide the therapeutic approach.
Pleural manometry is a general term referring to different methods of Ppl measurement used both in clinical practice and research studies. Techniques of Ppl measurement have evolved over the last 40 years, from the simple U-shaped water manometer to sophisticated electronic devices and single-use digital units. Nevertheless, no gold standard Ppl measurement technique exists with selection predominantly based on operator preference and equipment availability.
In this article, we present data on different pleural manometry techniques and discuss recent findings outlining potential clinical applications of Ppl measurement, highlighting their strengths and weaknesses.
Techniques of Pleural Pressure Measurement
Currently, four major categories of pleural manometers are used. These include simple U-tube water manometers, dedicated commercial digital manometers, simple manometry systems based on pressure transducers and ICU monitors, and complex, home-built, customized manometers based on hemodynamic electronic transducers [13, 14••, 15•, 16] (Figure 1 and Figure 2). Inter-category modifications exist, e.g., undampened and dampened water manometers [13, 15•, 16]. Customized electronic and commercial digital manometers appear to produce the most reproducible and accurate measurements and are favored for clinical practice [17••]. Basic characteristics, including the relative advantages and disadvantages of different manometry systems, are presented in Table 1.
Graphic depiction of basic data on different pleural manometry devices. The procedure of pleural pressure (Ppl) measurement requires the connection of the pleural catheter or chest tube to a manometry device. Apart from the simple water U-tube, a key element of all manometers is a pressure transducer. Both ICU monitor and digital manometers have a built-in signal converter. In the home-built manometer, a custom design analog-to-digital converter (ADC) is required.
Three different manometer types: A Digital handheld manometer, courtesy of Professor David J Feller-Kopman, Director in Bronchoscopy & Interventional Pulmonology, Johns Hopkins Hospital, Baltimore, MD, United States; B digital manometer, courtesy of Professor Najib Rahman, Consultant and Senior Lecturer in Respiratory Medicine, Oxford Respiratory Trials Unit, Nuffield Department of Medicine, Oxford, UK; C home-built digital manometer; enlarged photo of the transducer and analog-to-digital converter in the right upper corner. Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Poland.
Update on Clinical Applications of Pleural Manometry
Several comprehensive reviews on pleural manometry have been published [14••, 18, 19]. These papers discuss various aspects of the procedure outlining its historical background, techniques of Ppl measurement and data interpretation, current and potential future clinical applications in patients with pleural effusion and pneumothorax as well as related pitfalls.
Hereon in our article aims not to replicate prior publication but to focus on new data supplemented by the authors’ own experience, including results of studies completed in recent years.
We searched PubMed/MEDLINE, Cochrane Library, and ClinicalTrials.gov databases using the following search terms: “pleural manometry,” “pleural pressure,” and “pleural elastance,” Reference lists from publications on pleural manometry were also reviewed to find relevant papers. The time frame of the search was confined to the years 2017–2021.
We identified 24 articles which were primarily or partially related to pleural manometry. These papers included 11 clinical trials, with 4 randomized controlled trials (RCTs). At the time of article submission, one observational and one randomized interventional study are ongoing. Four additional articles were identified through reference lists, related articles suggested by search engines or were already known to the authors. No systematic reviews or meta-analyses were published in the last 4 years.
Pleural Manometry Data in Pleura Modelling (In Silico Models)
Therapeutic thoracentesis is a routine procedure performed to achieve symptomatic relief, mainly alleviation of pleural effusion associated dyspnea. Pleural fluid withdrawal is associated with a Ppl decrease; however, the relationship between the volume of pleural fluid removed and the magnitude of Ppl drop is complex, affected not only by the mechanical properties of the expanding lung but also by the mechanics of the chest wall, diaphragm, and mediastinal structures. The assessment of all these variables is impossible in vivo but can be mirrored using mathematical models built and empowered by clinical data. A previously developed complex model of a virtual patient, which included respiratory mechanics, gas exchange, and pulmonary circulation modules, has been upgraded to encompass a new pleural module. This enables study of the interactions between pleural fluid removal, respiratory mechanics, and gas exchange [20, 21]. This concept, developed using data from 32 real pleural manometry procedures, has facilitated the evaluation of factors affecting Ppl and arterial blood gas (ABG) changes associated with pleural fluid withdrawal. Utilizing this module, we found that the compliance of the rib cage and mediastinum, along with the nonlinearity of the dependence between the transpulmonary pressure and lung volume, are important factors affecting Ppl fluctuation during therapeutic thoracentesis [19]. The variability witnessed in arterial blood gas (ABG) measurements during therapeutic thoracentesis may also be explained with the decrease in Ppl accompanying pleural fluid withdrawal, improving perfusion of atelectatic lung areas. However, the effect of therapeutic thoracentesis largely depends on the rate of recruitment of these areas, and a lack of ventilation may result not only in a lack of improvement in ABG but potentially a detrimental effect on arterial blood oxygen tension. Effective hypoxic pulmonary vasoconstriction may protect against this disadvantageous phenomenon [20].
Bearing in mind these hypotheses, we believe that pleural manometry is a valuable clinical tool and may broaden clinical knowledge about pleural physiology and the mechanisms which underlie common pleural diseases such as pleural effusion and pneumothorax.
Unexpandable Lung
The term “unexpandable lung” refers to various conditions which result in the inability of the lung to expand to sanction normal pleural interaction. Three major causes of unexpandable lung have been recognized: (I) endobronchial obstruction resulting in lobar collapse or chronic lung atelectasis; (II) decreased lung compliance due to extensive pulmonary scarring and fibrosis; and (III) visceral pleural restriction secondary to pleural disease [13, 14••, 22].
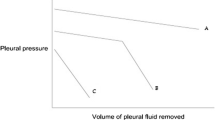
Depending on the nature of visceral pleural restriction, two types of unexpandable lung have been reported: trapped lung and lung entrapment. In patients with trapped lung, fluid removal is associated with a steep decline of Ppl and usually a negative initial Ppl. Lung entrapment allows a partial re-expansion of the lung in the initial phase of pleural fluid withdrawal and is usually characterized by a positive baseline Ppl. However, a normal or near-normal initial pattern of pleural pressure-volume curve changes along with the increasing volume of fluid removed reflects the inability of the lung to re-expand beyond a certain volume. This is depicted by a steeper decline of the second section of the pressure-volume curve as presented in Figure 3. The numerical relationship between the volume of fluid removed and the respective Ppl decline is referred to as pleural elastance (Pel). Neglecting the effect of factors discussed in the section on pleura modelling below, Pel can be calculated as a derivative of Ppl drop versus the volume of pleural fluid removed (dP/dV). Thus, Pel reflects the ability of the lung to re-expand after fluid or air withdrawal. Based on the results of 192 pleural manometry procedures during therapeutic thoracenteses, Heidecker et al defined normal Pel as ranging between 0.5 and 14.5 cmH2O/L (mean ± 2 SDs) and an elevated Pel as higher than 14.5 cmH2O/L) [23].
Diagnosis of Unexpandable Lung
The diagnosis of an unexpandable lung is important in determining the management of patients with malignant pleural effusion (MPE). Dependent on patients’ clinical context, predicted life expectancy, preferences, and the ability of the lung to re-expand, three therapeutic approaches can be offered: (1) serial thoracenteses, (2) pleurodesis, and (3) indwelling pleural catheter (IPC) insertion. Both pleurodesis and IPC are preferred as “definitive” procedures [24•] resulting in improvement of health-related quality of life [25] and reducing the risk of future pleural intervention. Assessment of lung expandability is crucial when pleurodesis is considered a therapeutic option as pleural apposition is a key prerequisite for effective pleural adhesion. Although pleural manometry is an elegant method to estimate Pel and the mechanical properties of the lung, lung expandability can also be assessed based on radiological signs of pneumothorax ex vacuo or a failure to achieve total lung re-expansion after pleural fluid withdrawal. Importantly, according to the statement of the British Thoracic Society (BTS), radiological evidence of complete lack of lung expansion and pleural apposition should prompt IPC insertion, not pleurodesis [26]. In this context, an interesting study comparing the relationship between Pel and post-thoracentesis radiographic findings has recently been published by Chopra et al [27••]. This study included 70 patients with MPE who underwent therapeutic thoracentesis with pleural manometry. The results of post-thoracentesis radiographs were analyzed in terms of Pel calculated based on pleural manometry data. Elevated Pel and incomplete post-procedure lung re-expansion were found in 36 (51.4%) and 38 (54%) patients, respectively. The concordance between post-procedure radiographic criteria for lung re-expansion pleural elastance was found in 50 cases only (71%). In 20 patients (29%), radiological findings did not match manometry results. Complete lung re-expansion was found in only 23/34 (68%) patients with normal Pel but also in 9/36 (25%) patients with elevated Pel. The results of this study point out that the prediction of lung expandability by pleural manometry results should be interpreted with caution. On the other hand, the authors concluded that, compared to post-thoracentesis chest radiograph, pleural manometry may have an additional role in selecting patients for pleurodesis and that this issue should be further evaluated in terms of pleurodesis outcomes.
Also, some studies suggest that the combination of manometry with other techniques can improve its diagnostic or therapeutic capabilities. Salamonsen et al [28] showed that the use of transthoracic ultrasound may increase the sensitivity of the diagnosis of unexpandable lung.
Direct Use of Pleural Manometry to Allocate Patients to Pleurodesis vs. IPC
The performance of pleural manometry and Pel to aid patient selection for talc pleurodesis vs. IPC was evaluated by Martin et al. In their small randomized feasibility trial, pre-EDIT (elastance-directed indwelling pleural catheter or talc slurry pleurodesis (TSP)), 31 patients were allocated to pleurodesis either with the application of pleural manometry or to standard care [27••]. Pleural elastance was successfully measured in 13 of 15 patients (87%) with elevated Pel detected in 7 of 13 patients allocated to the EDIT arm. The diagnosis of unexpandable lung based on Pel value equal or higher than 14.5 cm H2O/L was found to be 100% sensitive and 67% specific. Hence, the study showed that a phase 3 trial evaluating the impact of Pel-directed allocation of symptomatic patients with MPE to TSP is feasible. A further observation of the effect of EDIT management on symptomatic MPE recurrence following TSP is reasonable. [29•].
The role of pleural manometry and Pel calculation in the prediction of the success rate of doxycycline pleurodesis was recently highlighted by Massoud et al. The authors performed chemical pleurodesis in 40 patients and found that subjects with successful pleurodesis were characterized by significantly lower Pel than those who failed to achieve pleurodesis (8.38±2.65 vs. 18.29±4.65 cmH2O/L, respectively). ROC analysis showed that Pel > 14.5 cmH2O/L was a significant predictor of pleurodesis failure, with sensitivity, specificity, and AUC of 94%, 100%, and 0.94, respectively [30]. Based on the results of the above-presented studies, pleural manometry may be considered a useful tool to guide treatment selection (IPC vs. pleurodesis) for patients with MPE.
Less encouraging outcomes were presented by Halford et al. In their multicenter study, a sub-study of the IPC-PLUS trial [31], 89 of 250 patients in whom pleural manometry had been performed at IPC insertion were assessed. The authors aimed to address the feasibility and utility of Pel measurement via IPC (at the time of catheter insertion) as a predictor of lung expandability [32•]. The results of pleural manometry were compared with radiographic data at day 10 which were considered the diagnostic standard. The authors found that patients with substantial limitation of lung expansion had a significantly lower median closing Ppl (−15.00 vs. 0.00 cmH2O, p=0.012) and higher terminal Pel (12.03 vs. 8.59 cmH2O/L, p=0.021) compared to the remaining patients. However, the discriminative value of Pel in terms of detection of substantial lung unexpandability was poor with an AUC for closing Ppl and Pel 0.695 and 0.680, respectively. Therefore, the authors concluded that opening manometry was not useful in accurate predicting substantial lung unexpandability.
Pleural Manometry and Safety of Large-Volume Thoracentesis
Therapeutic thoracentesis may be associated with adverse reactions, including chest discomfort or pain, cough, iatrogenic pneumothorax, and re-expansion pulmonary edema (RPE). The risk of these complications is thought to be at least partially associated with the drop in Ppl itself determined by the volume of pleural fluid withdrawn and the mechanical properties of the pleural space, i.e., by Pel [33,34,35]. This suggests that intra-procedure Ppl monitoring may help to prevent adverse sequelae. Indeed, in an early study by Light et al, none of the patients in whom Ppl remained over −20 cm H2O developed serious thoracentesis-related complications [36]. As pleural manometry was not widely available at that time, the authors recommended the volume of withdrawn fluid should not exceed 1000 mL if Ppl monitoring is not conducted; this recommendation was later mitigated to 1500 mL a volume advocated by newer guidelines [9, 37].
Other studies evaluated the relationship between the adverse effects of large-volume thoracentesis and Ppl decline. Feller-Kopman et al demonstrated that chest discomfort was associated with a significant drop in Ppl and should be construed as a sign to terminate thoracentesis [33]. On the other hand, the same group demonstrated that RPE after large-volume thoracentesis is rare and independent of the volume of fluid removed, Ppl and Pel [34]. Based on their experience, these authors suggested that large pleural effusions should be drained completely as long as the patient does not develop chest discomfort and/or the end-expiratory Ppl does not drop below −20 cmH2O [34]. Similarly, a significant relationship between Ppl drop after pleural fluid withdrawal and the development of clinical symptoms was reported by Khosla and Kistler [38]. Thus, these studies advocate a role for pleural manometry in the prevention of complications following large-volume thoracentesis.
However, these opinions have been challenged by the results of a recent multi-center single-blind randomized controlled trial by Lentz et al [39••]. The authors evaluated clinical outcomes of large-volume therapeutic thoracentesis in 124 patients randomly assigned to receive either thoracentesis guided by symptoms and pleural manometry vs. thoracentesis guided by symptoms only (control group). There were no significant differences in the chest discomfort score (p=0.56) between both groups. Asymptomatic pneumothorax occurred in 6 (10%) out of 62 patients in the control group compared with none in the manometry group (p=0.01). These results suggest that the measurement of Ppl during large-volume thoracentesis does not alter procedure-related chest discomfort and do not support the routine use of this approach in everyday clinical practice.
This was the first randomized, well-designed study on pleural manometry to reduce chest discomfort associated with large-volume thoracentesis, and its results are worthy of consideration. However, they are also intriguing, and in the context of an overall negative study, the issue of the volume of pleural fluid withdrawn should be raised. In approximately 40% of patients, less than 1.0 L of fluid was evacuated. The removal of this modest volume may be an important factor contributing toward the study’s findings [40]. The authors of this review believe that if manometry is applied with the intention to prevent procedure-related complications, it should be offered to patients undergoing true large-volume thoracentesis (>1.0 or 1.5 L) and those with an elevated risk of trapped, e.g., patients with long-standing pleural effusion with inflammatory features and patients who have experienced dyspnea or chest pain during prior pleural fluid withdrawals. Considering these limitations, we believe that further studies should be undertaken to define selection criteria for those who may benefit from peri-thoracentesis pleural manometry.
Factors and Approaches to Reduce the Rate of Pleural Pressure Decline During Pleural Fluid Withdrawal
As an excessive decline of Ppl may be related to adverse reactions, it seems rational to search for factors and approaches that can decelerate the rate of pleural pressure decline. It might be presumed that application of positive airway pressure may increase transpulmonary pressure helping to re-open small obstructed bronchioles and alveoli. These effects could improve the ability of the lung to re-expand and lessen the acuity of the pleural pressure-volume curve. A small pilot study by Abouzgheib et al was performed to test this hypothesis [41•]. The authors compared the pattern of Ppl decrease during therapeutic thoracentesis in patients allocated to two arms. In the interventional arm, a continuous positive airway pressure (CPAP) of +5 cmH2O was applied via a CPAP mask to 25 patients undergoing pleural fluid withdrawal with pleural manometry. In patients allocated to the control group (n=24), the same procedure was performed without applying CPAP [41•]. Although the mean volumes of drained fluid were comparable in both groups (1380 and 1396 mL for CPAP and control group, respectively), a significantly greater decline in Ppl was found in the control arm: 17.4 (6 to 33) vs. 11.8 (4 to 21) cmH2O, respectively. Furthermore, contrary to the control group in which 8 (33%) patients developed a closing Ppl below −20 cmH2O, none of the CPAP patients had closing Ppl lower than −20 cmH2O. The results of the study demonstrate that application of CPAP during therapeutic thoracentesis increases the compliance of the pleural space and mitigates the Ppl response to thoracentesis. Although the sample size was small, this finding may have a real clinical application and warrants further study.
In the context of factors which can prevent an excessive Ppl decline during pleural fluid removal, an intriguing observation was made regarding cough. Cough associated with thoracentesis is usually considered an adverse reaction to changes in Ppl. However, our group found that spontaneous, thoracentesis-associated cough may also have a beneficial effect expressed by an elevation of Ppl [42]. An initial paper published in 2015 was based on 3 patients only and reported an increase of Ppl ranging from 1.4 to 3.1 cmH2O directly after coughing bouts [42]. The beneficial effect of cough has been echoed in a larger study currently under consideration for publication, which demonstrated that although cough was less common in patients with elevated Pel (p<0.0001), it was associated with significantly higher Ppl increase than in patients with normal Pel.
Other Findings in Patients with Pleural Effusion Made with the Use of Pleural Manometry
Using pleural manometry in the frame of one research project (NCT02192138), we noted that the Ppl curve showed small-amplitude oscillations resembling the pulse tracing line. We hypothesized that these small Ppl fluctuations were related to transmission of the cardiac pulsation to the atelectatic lung and subsequently the pleural fluid. This hypothesis was tested in 54 patients in whom simultaneous Ppl, ECG, and pulse (home-built photoplethysmography device) records were available [43]. We found that, using a sensitive electronic pleural manometer, measurement, and registration of instantaneous Ppl, pleural pressure oscillations can be detected in more than 80% of patients. Furthermore, the nadirs of Ppl waves were perfectly matched with the points on the ECG curve corresponding to the smallest ventricular volumes during the cardiac cycle. Therefore, we believe that these pleural pressure oscillations are caused by cyclic changes in the volume of heart chambers during systolic and diastolic phases. As the small cyclic changes in Ppl are associated with cardiac hemodynamics, we proposed the term “pleural pressure pulse” (PPP) to describe this phenomenon. The importance of PPP monitoring remains unknown, yet we suggest that its appearance in the baseline Ppl measurement during large-volume thoracentesis may indicate significant lung atelectasis or lower lung/visceral pleura compliance and thereby worse lung expandability. This assumption is based on similar observations of the “lung pulse,” a phenomenon first described by Lichtenstein et al [44] representing pulsations of the pleural line synchronized with the heart cycles in single lung intubated patients and also evident in healthy volunteers during breath-hold and apneas. It has been shown that the lung pulse might be a reliable marker of complete lung atelectasis.
Based on our observations, we propose that pleural manometry may be a more sensitive and more accurate measure of pleural pulsations, albeit more invasive, than M-mode US imaging [43]. Further studies involving simultaneous pleural ultrasound imaging and PPP measurements are warranted to test this hypothesis.
Pleural Manometry in Pneumothorax
There have been attempts to apply measurement of pleural pressure to patients with pneumothorax [45, 46]. Recently, Kaneda et al showed that the mean Ppl in patients with persistent air leak was lower than in patients without air leak (p=0.020 and p=0.006) [46]. These initial results seem promising; however, further studies are needed to evaluate the role of pleural manometry as a guide for decision-making in patients with pneumothorax.
One study showed that expiratory (but not inspiratory) Ppl was significantly lower in patients who were successfully managed with pleural aspiration or drainage compared to those who required surgical treatment [45]. Both inspiratory and expiratory Ppl were significantly lower in patients with complete pneumothorax resolution within 1 week of drainage than in patients who required longer treatment. A different study reported a potential role of pleural manometry for the identification of pressure-dependent pneumothorax after partial lung resection [47]. A similar diagnostic method for pneumothorax ex vacuo with the use of pleural manometry was proposed by Tan et al [48]. It can be presumed that the ability of air leak detection and differentiation between pressure-dependent and independent air leak may point to a new practical application of pleural manometry in the management of pneumothorax.
Currently, a prospective observatory trial (NCT04630301) is underway at Johns Hopkins University (Baltimore, MD, USA). The general concept of the study is assessment of clinical outcomes of patients undergoing procedures for pneumothorax and their correlation with pleural pressure.
Drawbacks and Limitations of Pleural Manometry
Despite the numerous technical and digital advancements, miniaturization, and larger accessibility, general use of pleural manometry remains limited predominantly to specialized centers and research settings [19]. Moreover, the methodology and techniques of pleural manometry have not been standardized [16]. Application of pleural manometry during therapeutic thoracentesis may be time-consuming and usually requires an additional nurse or technician to ensure the smooth and effective running of the procedure. As most of the systems use single lumen catheters, the measurement requires a pause in pleural fluid flow through the catheter and prolongation of the procedure by several to a dozen minutes, depending on the total number of measurements and the duration of the individual measurement.
Summary and Future Directions
Pleural manometry is a relatively simple measurement technique which can provide data on different aspects of pleural pathophysiology. Since pleural manometers have not been adequately popularized and are not widely available, the procedure is usually performed in specialized pulmonary centers, mainly for research purpose.
It remains crucial to define the precise role of pleural manometry in patients with different pleural diseases, including indications for the procedure, and despite promising results, there is insufficient data to support routine use of pleural manometry during large-volume thoracentesis.
Several new clinical applications of pleural manometry merit further evaluation including extension to its application in the management of spontaneous pneumothorax.
Thus, further studies on pleural manometry are needed and should address the following: (1) evaluation and verification of a “safe” Ppl threshold; (2) determination of an optimal phase of the respiratory cycle for Ppl measurement; (3) establishment of a valid method of Pel calculation; (4) the need to expand knowledge on pathophysiological processes in the pleural cavity and their influence on respiratory, cardiovascular, and neuromuscular systems in patients with pleural effusion and pneumothorax; and (5) application research data and observations to bedside clinical practice [19, 28, 34, 39, 42, 49•, 50].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Krenke R, Mierzejewski M. Anatomy and physiology of the pleural space. Reference Module in Biomedical Sciences. 2020. https://doi.org/10.1016/B978-0-12-801238-3.11577-6A recent comprehensive review of the current knowledge about the anatomy, physiology and pathophysiology of the pleura.
Akulian J, Yarmus L, Feller-Kopman D. The evaluation and clinical application of pleural physiology. Clin Chest Med. 2013;34(1):11–9. https://doi.org/10.1016/j.ccm.2012.11.001.
Feller-Kopman D, Parker MJ, Schwartzstein RM. Assessment of pleural pressure in the evaluation of pleural effusions. Chest. 2009;135(1):201–9. https://doi.org/10.1378/chest.08-1788.
Light RW. Mechanics of respiration. In: George RB, Light RW, Matthay MA, Matthay RA, editors. Chest medicine. Essentials of pulmonary and critical care medicine. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 24–38.
McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):48S–53S. https://doi.org/10.1378/chest.129.1_suppl.48S.
Gaensler EA. Parietal pleurectomy for recurrent spontaneous pneumothorax. Surg Gynecol Obstet. 1956;102(3):293–308.
Ukale V, Bone D, Hillerdal G, Cederlund K, Widström O, Larsen F. The impact of pleurodesis in malignant effusion on respiratory function. Respir Med. 1999;93(12):898–902. https://doi.org/10.1016/s0954-6111(99)90056-2.
West JB. Why doesn't the elephant have a pleural space? News Physiol Sci. 2002;17:47–50. https://doi.org/10.1152/nips.01374.2001.
Light RW. Pleural Diseases. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis. J Bronchol Intervent Pulmonol. 2014;21(4):306–13. https://doi.org/10.1097/lbr.0000000000000095The authors showed that the measurement of pleural pressure is not predictive of development of chest discomfort during therapeutic thoracentesis.
Duszak R, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–64. https://doi.org/10.1016/j.jacr.2010.04.013.
Hallifax RJ, Goldacre R, Landray MJ, Rahman NM, Goldacre MJ. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320(14):1471–80. https://doi.org/10.1001/jama.2018.14299The most recent study on the epidemiology of spontaneous pneumothorax.
Grabczak EM, Krenke R. Pleural interventions. Manometry. Reference Module in Biomedical Sciences, 2020; https://doi.org/10.1016/B978-0-12-801238-3.11566-1.
Hu K, Chopra A, Huggins JT, Nanchal R. Pleural manometry: techniques, applications, and pitfalls. J Thorac Dis. 2020;12(5):2759–70. https://doi.org/10.21037/jtd.2020.04.04An excellent review on different methods of pleural pressure measurement, applications of pleural manometry and its limitations.
Salamonsen M, Ware R, Fielding D. A new method for performing continuous manometry during pleural effusion drainage. Respiration. 2014;88(1):61–6. https://doi.org/10.1159/000358842This is the first paper presenting continuous pleural pressure measurement and reported no difference in pleural elastance measured by traditional and new methods.
Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764–9. https://doi.org/10.1378/chest.126.6.1764.
Lee HJ, Yarmus L, Kidd D, Ortiz R, Akulian J, Gilbert C, et al. Comparison of pleural pressure measuring instruments. Chest. 2014;146(4):1007–12. https://doi.org/10.1378/chest.13-3004The authors compared the results of pleural pressure measurement with U-tube water manometer, handheld digital manometer and an electronic transducer. The digital manometer was shown to be a reliable and valid tool for pleural pressure measurement.
Grabczak EM, Krenke R, Zielinska-Krawczyk M, Light RW. Pleural manometry in patients with pleural diseases – the usefulness in clinical practice. Respir Med. 2018;145:230–6. https://doi.org/10.1016/j.rmed.2018.01.014.
Zielinska-Krawczyk M, Krenke R, Grabczak EM, Light RW. Pleural manometry–historical background, rationale for use and methods of measurement. Respir Med. 2018;136:21–8. https://doi.org/10.1016/j.rmed.2018.01.013.
Golczewski T, Stecka AM, Michnikowski M, Grabczak EM, Korczynski P, Krenke R. The use of a virtual patient to follow pleural pressure changes associated with therapeutic thoracentesis. Int J Artif Organs. 2017;40(12):690–5. https://doi.org/10.5301/ijao.5000636.
Stecka AM, Golczewski T, Grabczak EM, Zielinski K, Michnikowski M, Zielimska-Krawczyk M, et al. The use of a virtual patient to follow changes in arterial blood gases associated with therapeutic thoracentesis. Int J Artif Organs. 2018;41:690–7. https://doi.org/10.1177/0391398818793354.
Huggins JT, Maldonado F, Chopra A, Rahman N, Light R. Unexpandable lung from pleural disease. Respirology. 2018;23(2):160–7. https://doi.org/10.1111/resp.13199.
Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173–84. https://doi.org/10.1378/chest.130.4.1173.
Bibby AC, Dorn P, Psallidas I, Porcel JM, Janssen J, Froudarakis M, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1):1800349. https://doi.org/10.1183/13993003.00349-2018The paper provides answers to 6 important questions on the management of malignant pleural effusion including its definitive treatment with pleurodesis and indwelling pleural catheter.
Sivakumar P, Saigal A, Ahmed L. Quality of life after interventions for malignant pleural effusions: a systematic review. BMJ Support Palliat Care. 2018;10(1):45–54. https://doi.org/10.1136/bmjspcare-2018-001610.
Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii32–40. https://doi.org/10.1136/thx.2010.136994.
Chopra A, Judson MA, Doelken P, Maldonado F, Rahman NM, Huggins JT. The relationship of pleural manometry with postthoracentesis chest radiographic findings in malignant pleural effusion. Chest. 2020;157(2):421–6. https://doi.org/10.1016/j.chest.2019.08.1920The study provides important data on some discordance between the diagnosis of the incomplete lung expansion after pleural fluid withdrawal based on pleural elastance calculation and post-thoracentesis radiograph.
Salamonsen MR, Lo AKC, Ng ACT, Bashirzadeh F, Wang WYS, Fielding DIK. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146(5):1286–93. https://doi.org/10.1378/chest.13-2876.
Martin GA, Tsim S, Kidd AC, Foster JE, McLoone P, Chalmers A, et al. Pre-EDIT: a randomized feasibility trial of elastance-directed intrapleural catheter or talc pleurodesis in malignant pleural effusion. Chest. 2019;156(6):1204–13. https://doi.org/10.1016/j.chest.2019.07.010The authors performed a feasibility study on management of malignant pleural effusion with elastance-directed indwelling pleural catheter vs. talc slurry. The study demonstrated that the measurement of pleural elastance with a novel digital manometer might be a guide to choosing a definitive treatment option.
Masoud HH, El-Zorkany MM, Ahmed AA, Assal HH. Pleural space elastance and its relation to success rates of pleurodesis in malignant pleural effusion. Tuberc Respir Dis. 2021;84(1):67–73. https://doi.org/10.4046/trd.2020.0081.
Bhatnagar R, Keenan EK, Morley AJ, Kahan BC, Stanton AE, Haris M, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378(14):1313–22. https://doi.org/10.1056/nejmoa1716883.
Halford PJ, Bhatnagar R, White P, Haris M, Harrison RN, Holme J, et al. Manometry performed at indwelling pleural catheter insertion to predict unexpandable lung. J Thorac Dis. 2020;12(4):1374–84. https://doi.org/10.21037/jtd.2020.02.25The study included the measurement of pleural pressure at indwelling pleural catheter insertion to predict unexpandable lung. Poor predictive ability of the pleural elastance to detect significant disturbances in lung re-expansion after pleural fluid withdrawal was found.
Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556–60. https://doi.org/10.1378/chest.129.6.1556.
Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large volume thoracentesis and the risk of re-expansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656–61. https://doi.org/10.1016/j.athoracsur.2007.06.038.
Pereyra MF, Ferreiro L, Valdes L. Unexpandable lung. Arch Bronconeumol. 2013;49(2):63–9. https://doi.org/10.1016/j.arbres.2012.05.007.
Light RW, Jenkinson SG, Minh VD, George RB. Observations on pleural fluid pressures as fluid is withdrawn during thoracentesis. Am Rev Respir Dis. 1980;121(5):799–804. https://doi.org/10.1164/arrd.1980.121.5.799.
Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii61–76. https://doi.org/10.1136/thx.2010.137026.
Khosla R, Kistler CR. Correlation between symptoms and complication to closing pleural pressure during thoracentesis. Am J Respir Crit Care Med. 2013; https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference. 2013.187.1_MeetingAbstracts.A4302
Lentz RJ, Lerner AD, Pannu JK, Merrick CM, Roller L, Walston C, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7(5):447–55. https://doi.org/10.1016/s2213-2600(18)30421-1This is the first randomized controlled trial showing that a routine use of pleural manometry during therapeutic thoracentesis does not alter procedure-related chest discomfort and suggesting no benefit from routine measurement of pleural pressure during the procedure.
Krenke R, Grabczak EM. Pleural manometry and thoracentesis is the issue resolved? Lancet Respir Med. 2019;7(5):374–6. https://doi.org/10.1016/S2213-2600(19)30033-5.
Abouzgheib W, Arya R, Cruz-Morel K, Koleman D, Kass J, Boujaoude Z, et al. The impact of continuous positive airway pressure upon pleural fluid pressures during thoracentesis. Respiration. 2019;98(1):55–9. https://doi.org/10.1159/000496610An interesting paper reporting that the application of CPAP is associated with the increase of pleural compliance and slowing down of the rate of pleural pressure decline during therapeutic thoracentesis.
Zielinska-Krawczyk M, Michnikowski M, Grabczak EM, Palko KJ, Korczynski P, Golczewski T, et al. Cough during therapeutic thoracentesis: friend or foe? Respirology. 2014;20(1):166–8. https://doi.org/10.1111/resp.12426.
Grabczak EM, Michnikowski M, Styczynski G, Zielinska-Krawczyk M, Stecka AM, Korczynski P, et al. Pleural pressure pulse in patients with pleural effusion: a new phenomenon registered during thoracentesis with pleural manometry. J Clin Med. 2020;9(8):2396. https://doi.org/10.3390/jcm9082396.
Lichtenstein DA, Lascols N, Prin S, Meziere G. The “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive Care Med. 2003;29(12):2187–92. https://doi.org/10.1007/s00134-003-1930-9.
Herrejon A, Inchaurraga I, Vivas C, et al. Initial pleural pressure easurement in spontaneous pneumothorax. Lung. 2000;178(5):309–16. https://doi.org/10.1007/s004080000034.
Kaneda H, Nakano T, Murakawa T. Measurement of intrapleural pressure in patients with spontaneous pneumothorax: a pilot study. BMC Pulm Med. 2019;19(1):267. https://doi.org/10.1186/s12890-019-1038-9.
Chopra A, Doelken P, Judson MA, Huggins T. The pressure-dependent air leak after partial lung resection. Thorax. 2017;72(3):290–1. https://doi.org/10.1136/thoraxjnl-2016-208884.
Tan GP, Tien KMCH, Chai GT, Abisheganaden JA. Simple pleural manometry using existing pleural drainage system: Experience of two cases of pleural effusions with post-drainage unexpandable lung and pneumothorax ex vacuo. Respirology. 2017;22:42. https://doi.org/10.1111/resp.13206_101.
Bhatnagar R, Corcoran JP, Maldonado F, Feller-Kopman D, Janssen J, Astoul P, et al. Advanced medical interventions in pleural disease. Eur Respir Rev. 2016;25(140):199–213. https://doi.org/10.1183/16000617.0020-2016A comprehensive review on various pleural interventions, including the therapeutic approach to unexpandable lung.
Maldonado F, Feller-Kopman D. Should manometry be routinely used during thoracentesis? Yes, but not without some basic physiologic understanding! Expert Rev Respir Med. 2016;10(10):1035–7. https://doi.org/10.1080/17476348.2016.1227248.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Katarzyna Faber declare no conflict of interest. Rafal Krenke reports grant from the National Science Centre, Poland, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pleural Diseases and Mesothelioma
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Faber, K., Krenke, R. Pleural Manometry—Basics for Clinical Practice. Curr Pulmonol Rep 10, 111–120 (2021). https://doi.org/10.1007/s13665-021-00277-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-021-00277-z